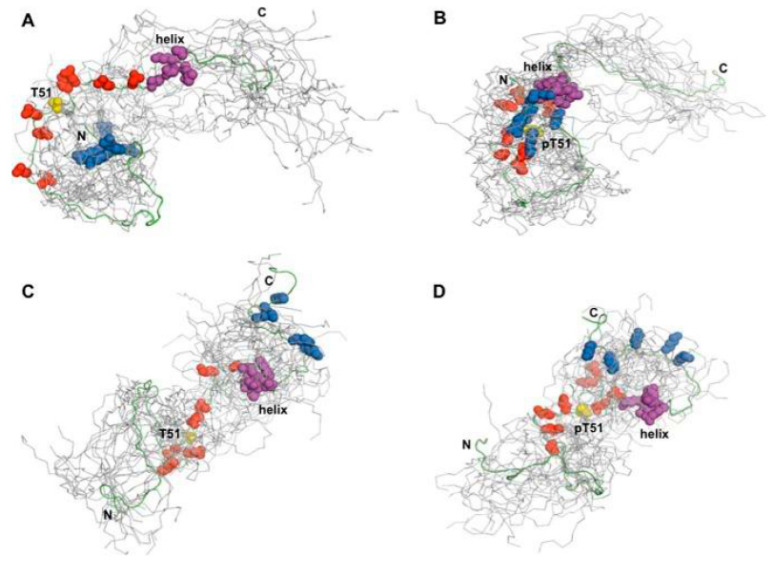
Figure 3.
Model for phosphorylation-induced conformational ensemble switching in PAGE4. (A), the non-phosphorylated PAGE4 adopts preferred transient structures such as the one highlighted from an ensemble of the 20 lowest energy conformers, where, on average, the N-terminal basic motif (blue spheres; Arg-4, Arg-6, Arg-8, Arg-10, and Arg-12) interacts weakly with the central acidic region (red spheres; Glu-43, Glu-47, Glu-49, Glu-55, Glu-56, Glu-60, and Asp-62) neighboring Thr-51 (yellow). (B), upon phosphorylation at Thr-51, the central region becomes more compact and more negatively charged, decreasing the average distance between Thr(P)-51, the basic motif, and the transient helix (magenta). (C) and (D), models of the transient interaction between the central acidic region and the C-terminal basic motif (blue spheres; Lys-82, Lys-84, Lys-90, Lys-93, and Lys-95) in non-phosphorylated PAGE4 (C) and Thr(P)-51 PAGE4 (D). The total number of distance restraints used was as follows: (A), 51; (B), 55; (C), 53; (D), 61. Adopted from [40].