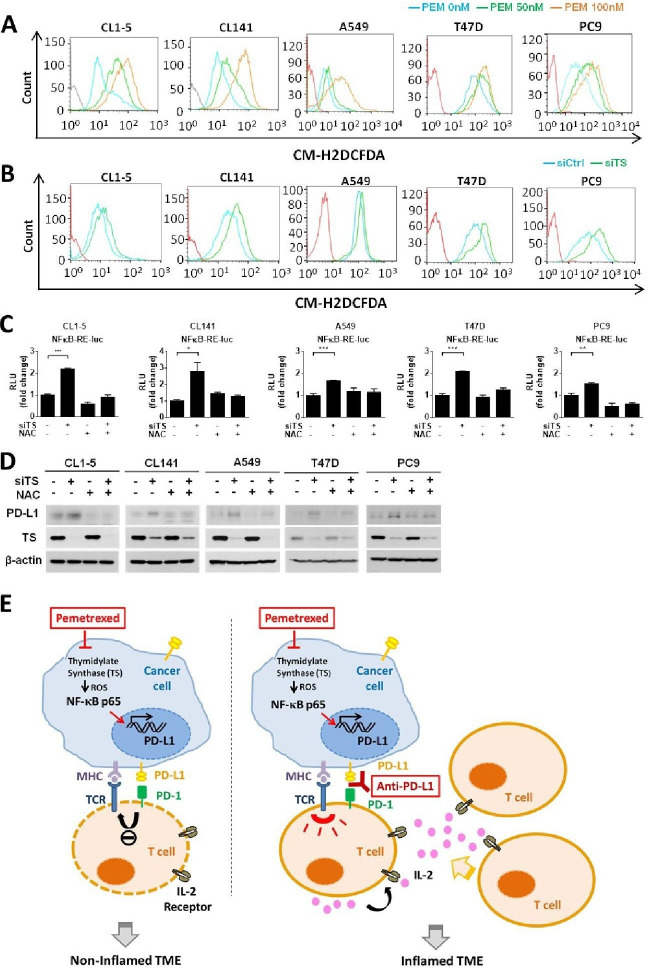
Figure 6.
The thymidylate synthase (TS)−reactive oxygen species (ROS)−NF-κB regulatory axis mediates pemetrexed (PEM)-induced programmed death-ligand 1 (PD-L1) upregulation. (A, B) CL1-5, CL141, A549, T47D or PC9 cells were treated with PEM (50 or 100 nM) alone or in combination with control siRNA (siCtrl) or TS-siRNA (siTS) for 24 hours, followed by incubation with the DCFDA fluorogenic dye (10 µM) for 30 min. The levels of ROS were assessed by flow cytometry. (C) CL1-5, CL141, A549, T47D or PC9 cells transfected with the NF-κB−Luc reporter plasmid in combination with control siRNA (siCtrl) or TS-siRNA (siTS) were treated with N-acetylcysteine (NAC) (5 mM) or left untreated, for 72 hours. The luminescence signal from the NF-κB–Luc reporter (firefly luciferase) was normalized to that of cotransfected pRL-TK vector (Renilla luciferase) to control for transfection efficiency and was expressed as relative light units. Data are shown as means and SD for three independent experiments (n=3). (D) CL1-5, CL141, A549, T47D or PC9 cells transfected with control siRNA (siCtrl) or TS-siRNA (siTS) were treated with NAC (5 mM) or left untreated, for 72 hours. Cells were lysed and analyzed by immunoblotting with the indicated antibodies. (E) Proposed model illustrating the molecular basis of the combinational treatment of PEM and anti-PD-L1 antibody in non-small-cell lung cancer (NSCLC). PEM induces the inhibition of TS, which in turn increases intracellular levels of ROS and results in the activation of NF-κB signaling pathway. NF-κB then transactivates PD-L1 expression and increases the levels PD-L1 on the cell surface of tumor cells. The upregulation of PD-L1 expression in tumor cells may suppress T-cell activation through the PD-L1/PD-1 engagement and lead to non-T-cell-inflamed tumor microenvironment (left). However, the combinational treatment of PEM and anti-PD-1/PD-L1 antibodies reactivates T-cell activities and improves effector function (right). MHC, major histocompatibility complex; TCR, T-cell receptor; IL-2, Interleukin-2; TME, tumor microenvironment.