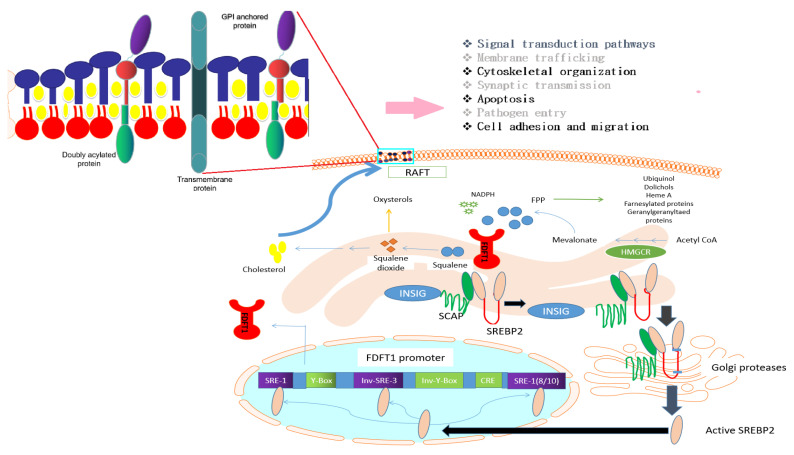
Figure 2.
Role of FDFT1 in the biosynthesis of cholesterol, structure and roles of lipid rafts and regulation of FDFT1 by SREBP2. FDFT1 is the first enzyme of the cholesterol biosynthesis branch, as FDFT1 converts farnesyl pyrophosphate (FPP) into squalene which ultimately produces cholesterol and cholesterol derivatives. Therefore, inhibition of FDFT1 only impacts the cholesterol biosynthesis pathway but not an alternative pathway, where FPP is converted into other essential cellular products such as ubiquinol, dolichols, heme A, farnesylated proteins, or geranylgeranylated proteins. Cholesterol is one of the most critical components of the plasma membrane microdomain known as rafts. Beyond cholesterol, the raft is structured by transmembrane proteins, doubly acylated proteins, and glycosylphosphatidylinositol (GPI) anchored proteins. Lipid rafts contribute to a variety of cellular biological processes such as signal transduction pathways, membrane trafficking, cytoskeletal organization, synaptic transmission, apoptosis, pathogen entry, cell adhesion, and migration. One of the key transcriptions factors of FDFT1 is SREBP2. SREBP2 is located in the endoplasmic reticulum (ER) membrane as an inactive form which is bound with Insulin Induced Gene 1 (INSIG) and Sterol Regulatory Element-Binding Protein Cleavage-Activating Protein (SCAP). Upon specific signals such as cholesterol deprivation, PI3K/AKT/mTOR, hypoxia, low pH, androgens, or ER stress, SREBP2 is activated by removal of INSIG from the complex INSIG-SCAP-SREBP2, and then SCAP-SREBP2 is transported to the Golgi, where SREBP2 is cleavage by Golgi proteases to form active SREBP2. The active SREBP2 form translocates into the nucleus and binds to SRE regions in the FDFT1 promoter including sterol regulatory element (SRE)-1, Inv SRE-3, and SRE-1 (8/10), which results in transcription of FDFT1.