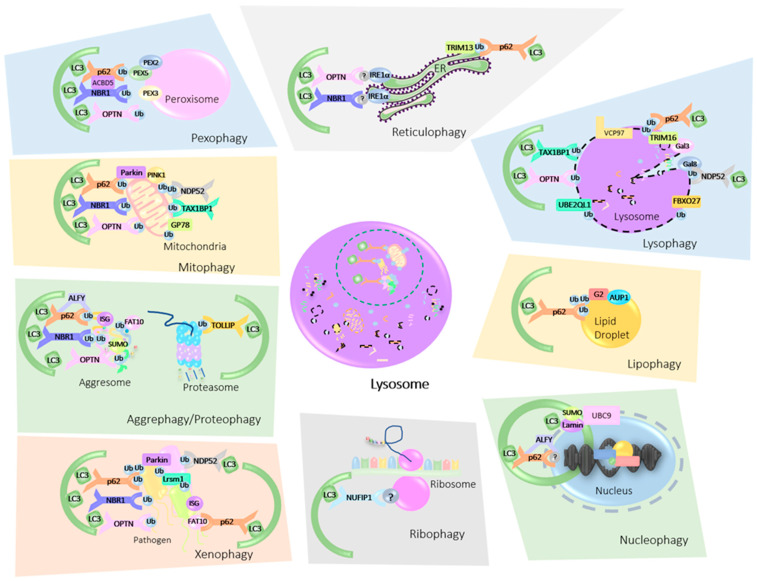
Figure 2.
Mammalian Ub-mediated Organellophagy. Damaged, dysfunctional or otherwise superfluous organelles are tagged for degradation by ubiquitin (Ub) or ubiquitin-like molecular modifiers (SUMO, FAT10, ISG15). These post translational modifications (PTMs) are then recognized by autophagy cargo receptors (p62, NBR1, OPTN, NDP52, TAX1BP1, NUFIP1) which bind both the ubiquitinated cargo and the microtubule-associated protein 1B light chain 3B (LC3B). The autophagosome engulfs the receptor cargo complex and delivers it to the lysosome for breakdown. ER-Phagy/Reticulophagy: ER stress sensor IRE1α, is activated upon ER stress. IRE1α interacts with p62, OPTN, and NBR1 to promote reticulophagy, but whether Ub is involved in this process is unclear. Self-ubiquitination of the E3 ligase TRIM13 also recruits p62 to damaged ER. This results in the sequestration of the ER within autophagosomes and its delivery to the lysosome for elimination. Pexophagy: PEX2 ubiquitinates the peroxisomal import receptor PEX5 which is then recognized by autophagy receptors p62 and NBR1. PEX3 is also involved in this process and enhances peroxisomal ubiquitination, while peroxisomal acyl-CoA binding domain containing 5 (ACBD5) regulates the pexophagy receptor protein complex. OPTN was also shown to be involved in this process. Mitophagy: Mitochondrial damage results in the stabilization of PINK1 on the mitochondrial outer membrane, which entices the arrival of E3 ligases Parkin and GP78. These ligases, in turn, ubiquitinate a variety of substrates on the mitochondrial membrane which recruits autophagy receptors p62, NDP52, OPTN, NBR1, and TAX1BP1. Aggrephagy/Proteophagy: Protein aggregates are recognized by selective autophagy receptors p62, NBR1, OPTN, and TOLLIP, with autophagy-linked FYVE protein (ALFY) acting as a scaffold protein promoting the assembly of p62 bodies. Cue5 the yeast homolog of mammalian TOLLIP binds ubiquitinated proteasomes targeting them for proteophagy. Ubiquitin and ubiquitin like PTMs (UBLs SUMO, FAT10, and ISG15) also play a role in aggrephagy. Xenophagy: The E3 ligases Parkin and Lrsm1 ubiquitinate invading pathogens which are then detected by p62, NBR1, NDP52, and OPTN. Other UBLs have also been demonstrated to promote xenophagy including FAT10 and ISG15. With FAT10 interacting directly with p62. Recognition by cargo receptors then promotes the incarceration of pathogens within autophagosomes and their delivery to lysosomes for elimination. Ribophagy: The large ribosomal subunit is targeted for autophagosomal degradation by the cargo receptor NUFIP1, however, the involvement of Ub or other UBLs in this process has not been determined. Nucleophagy: The selective autophagy receptor p62 and autophagic adaptor ALFY, have been documented to shuttle between the nucleus and cytoplasm, however whether Ub is involved in this process is not known. SUMOylation, was documented to drive nucleophagy where DNA damage promotes the accumulation of SUMO E2 ligase UBC9, resulting in the SUMOylation of lamin A/C. Lipophagy: Ancient Ubiquitous Protein 1 (AUP1) localizes to LDs and recruits the E2 ubiquitin-conjugating enzyme G2, which ubiquitinates targets on the lipid droplet, thus inviting autophagy receptor p62. Lysophagy: Glycans are exposed following lysosomal membrane rapture and are identified by galectins-3 and -8 (Gal3/8) or by the SCF ubiquitin ligase FBXO27. Gal3 then stimulates ubiquitination by TRIM16 and recruits the autophagy receptor p62, while Gal8 engages NDP52. The E2 ubiquitin-conjugating enzyme UBE2QL1 was also found to participate in this process, as were autophagy receptors TAX1BP1 and OPTN. The AAA-ATPase VCP97 is another essential player in the elimination of injured lysosomes.