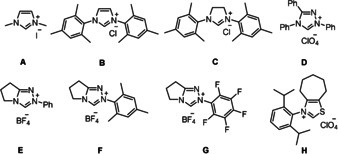
Table 1.
Optimization of the reaction conditions[a]
|
Entry |
NHC Precursor |
Photoredox Catalyst |
Light Source |
Yield[b] [%] |
|---|---|---|---|---|
|
1 |
A |
4CzIPN |
Blue LEDs |
trace |
|
2 |
B |
4CzIPN |
Blue LEDs |
4 |
|
3 |
C |
4CzIPN |
Blue LEDs |
2 |
|
4 |
D |
4CzIPN |
Blue LEDs |
3 |
|
5 |
E |
4CzIPN |
Blue LEDs |
15 |
|
6 |
F |
4CzIPN |
Blue LEDs |
36 |
|
7 |
G |
4CzIPN |
Blue LEDs |
7 |
|
8 |
H |
4CzIPN |
Blue LEDs |
11 |
|
9 |
F |
[Ir(dF(CF3)ppy)2(dtbbpy)]PF6 |
Blue LEDs |
32 |
|
10 |
F |
[Ir(ppy)2(dtbbpy)]PF6 |
Blue LEDs |
39 |
|
11 |
F |
[Ir(ppy)2(dtbbpy)]PF6 |
CFL |
44 |
|
12 |
F |
[Ir(ppy)2(dtbbpy)]PF6 |
CFL |
69[c] |
|
13 |
F |
[Ir(ppy)2(dtbbpy)]PF6 |
CFL |
80(74)[d] |
|
14 |
F |
[Ir(ppy)2(dtbbpy)]PF6 |
– |
0 |
|
15 |
– |
[Ir(ppy)2(dtbbpy)]PF6 |
CFL |
0 |
|
16 |
F |
– |
CFL |
0 |
|
|
|
|
|
|
|
| ||||
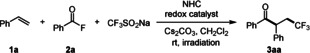
[a] Unless otherwise noted, all the reactions were carried out with benzoyl fluoride (0.3 mmol), styrene (0.15 mmol), CF3SO2Na (0.2 mmol), NHC (0.02 mmol), base (0.2 mmol), and 4CzIPN (0.002 mmol) in anhydrous CH2Cl2 (2 mL), irradiation with blue LEDs at room temperature for 24 h. [b] GC‐FID yield using 1,3,5‐trimethoxybenzene as an internal standard, yield of isolated product is given in parentheses. [c] 0.1 mmol of styrene was used. [d] 0.4 mmol of benzoyl fluoride was used. NHC=N‐Heterocyclic carbenes. 4CzIPN=2,4,5,6‐tetra(carbazol‐9‐yl)isophthalonitrile. CFL=compact fluorescent lamp.