Figure 5.
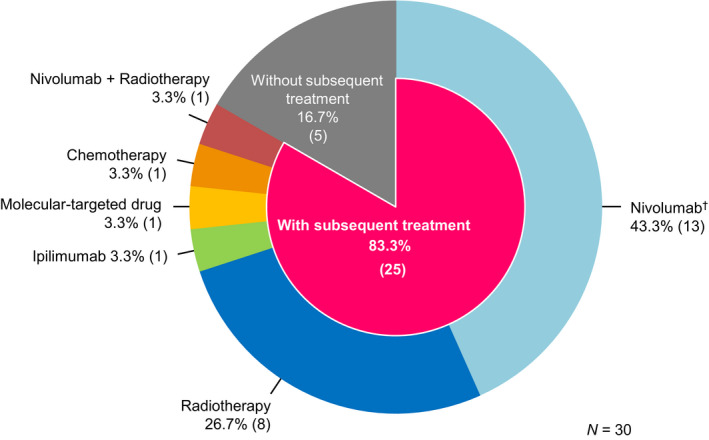
Subsequent therapy just after investigational agent discontinuation. †Four of 13 patients continued administration of nivolumab as a commercially available drug after acquisition of marketing approval.

Subsequent therapy just after investigational agent discontinuation. †Four of 13 patients continued administration of nivolumab as a commercially available drug after acquisition of marketing approval.