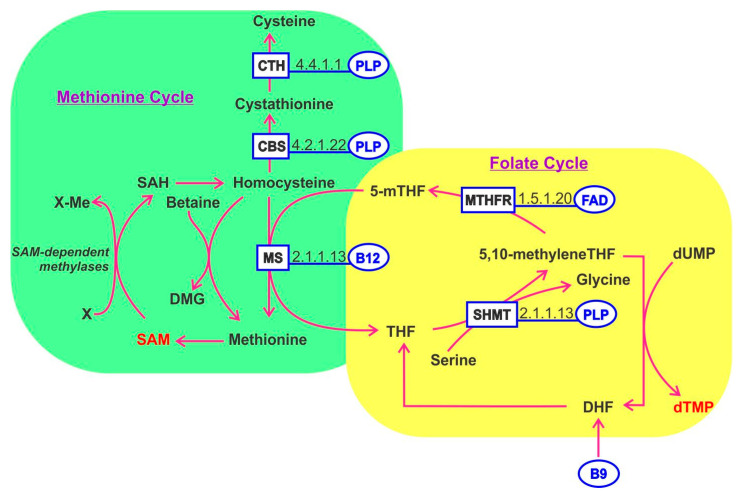
Figure 3.
The methionine and folate cycle pathways involving PLP-, FAD- and B12-dependent enzymes. The folate cycle begins with the conversion of dietary folate (B9) into dihydrofolate (DHF), which is then reduced to tetrahydrofolate (THF) by the enzyme dihydrofolate reductase (DHFR). THF is next converted to 5,10-methyleneTHF by serine hydroxymethyltransferase (SHMT), a reaction that is coupled with the hydroxylation of serine (Ser) to glycine (Gly) and requires PLP as a cofactor. Thymidylate synthase (TS) uses 5,10-methyleneTHF as a methyl donor to methylate deoxyuridine monophosphate (dUMP), creating deoxythymidine monophosphate (dTMP). This step regenerates DHF for continued cycling. Alternatively, 5,10-methyleneTHF can be reduced by methylenetetrahydrofolate reductase (MTHFR) to 5-methytetrahydrofolate (5-mTHF) using FAD as a cofactor. As part of the methionine cycle, 5-mTHF donates a methyl group to regenerate methionine from homocysteine (Hcy), which is catalyzed by methionine synthase (MS) that requires B12, in the form of methylcobalamin, as a cofactor. To generate the methyl donor S-adenosylmethionine (SAM) for use by multiple methyltransferases. SAM is demethylated during the methyltransferase reactions to form S-adenosylhomocysteine (SAH) which is then hydrolysed by S-adenosylhomocysteine hydrolase (AHCY) to form Hcy. Hcy can also enter the trans-sulfuration pathway catalyzed by cystathionine beta synthase (CBS) and cystathionine gamma lyase (CTH), both requiring PLP as a cofactor, to create cysteine.