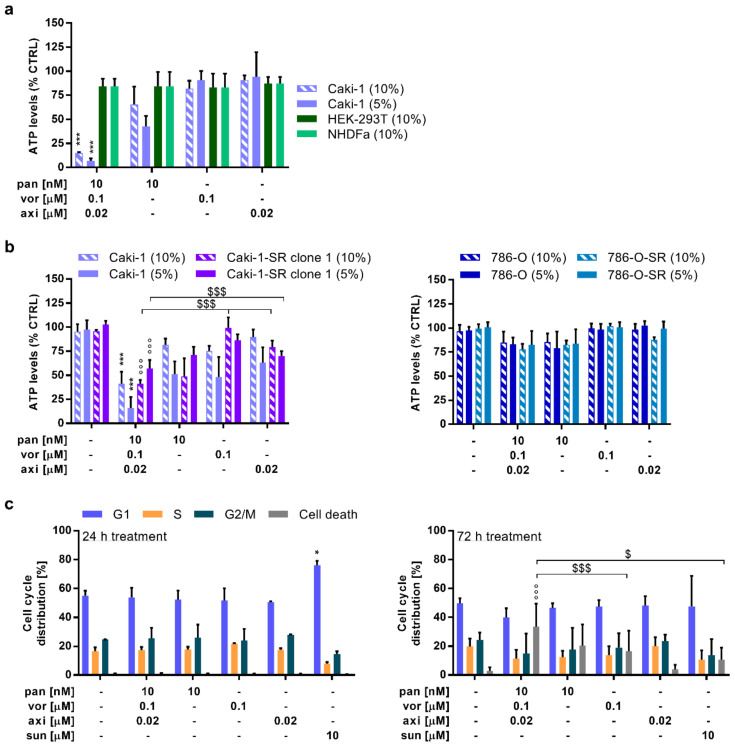
Figure 2.
Assessment of the optimized drug combination (ODC) activity: selectivity profile, the efficacy to overcome sunitinib-acquired resistance, and the induction of cell death. (a) Cross-validation of the multidrug combination and the corresponding monotherapies in Caki-1 cells and non-malignant HEK-293T cells, and human fibroblasts (NHDFα). The treatment was applied on Caki-1 cells in medium supplemented with either 5% or 10% fetal bovine serum and on the non-malignant cell lines with 10% fetal bovine serum. (N = 3) (b) Validation of the anticancer activity in sunitinib-resistant (-SR) Caki-1 cells, as well as primary ccRCC cell line 786-O and 786-O-SR cells. (N = 3) (c) Flow cytometry analysis elucidating G1, S, G2/M phase, and cell death with DNA binding propidium iodide (PI) after treating Caki-1 cells for 24 (left graph) and 72 h (right graph). (N = 3) Error bars represent the SD and the significance of estimated regression coefficients was determined with a one-way ANOVA and is represented with * p < 0.05 and *** p < 0.001 versus the CTRL (0.03% DMSO in culture medium) and the monotherapies. °°° p < 0.001 represents significance calculated versus the CTRL only whereas $ p < 0.05 and $$$ p < 0.001 represent significance calculated vs. the monotherapies only.