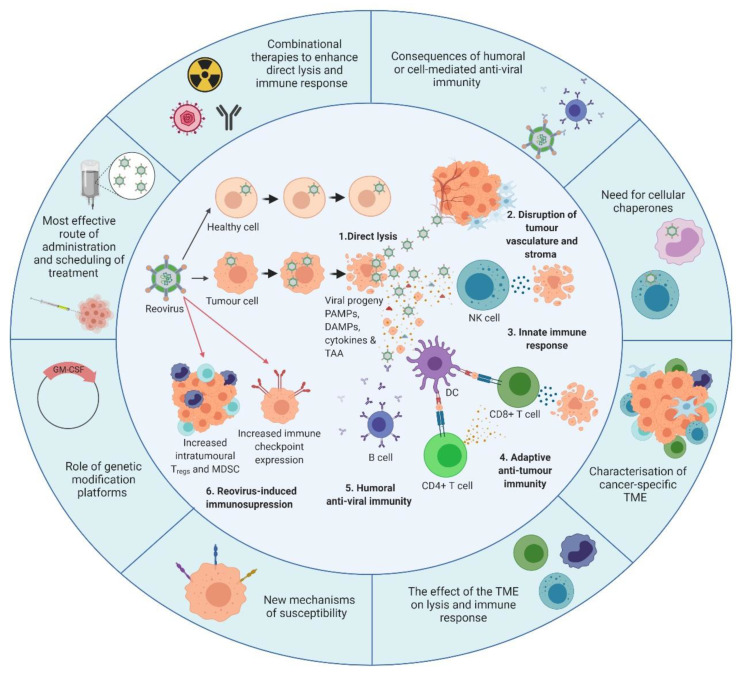
Figure 2.
Overview of reovirus mechanisms of action and the developments required. The inner circle illustrates what is currently known about reovirus. 1. In healthy cells, anti-viral immune responses limit reovirus replication and prevent lytic killing. By contrast, oncogenic signalling pathways render tumour cells susceptible to reovirus replication and direct oncolysis. 2. Reovirus replicates in the tumour vasculature and stroma due to reciprocal cell:cell interactions which alter anti-viral signalling. 3. Infection of tumour cells leads to the release of viral progeny, cytokines and tumour-associated antigens (TAAs), which initiates innate anti-tumour immunity including cytokine-mediated bystander killing and natural killer (NK) cell-mediated cytotoxicity. 4. Adaptive anti-tumour immunity is generated following the phagocytosis of TAAs by dendritic cells (DCs) and presentation of TAAs to CD4+ve and CD8+ve T cells, which facilitates priming of tumour-specific cytotoxic T lymphocytes (CTLs). 5. In addition to innate and adaptive anti-tumour immune responses, humoral anti-viral immunity is induced, leading to the production of reovirus-specific neutralising antibodies (NAbs). 6. Following induction of anti-tumour/anti-viral immune responses, regulatory immune mechanisms are “switched-on” to control ongoing immune responses, including upregulation of immune checkpoints and increased levels of regulatory T cells (Tregs) and/or myeloid-derived suppressor cells (MDSCs). The outer circle highlights priority research areas to improve reovirus efficacy. These include gaining a greater understanding of: (i) the consequence of humoral and/or cell-mediated anti-viral immunity on reovirus efficacy which would inform the development of, or the requirement for, cellular chaperones; (ii) the tumour microenvironment (TME) and how it influences reovirus oncolysis and anti-tumour immunity; (iii) the cellular determinants utilized by reovirus for direct oncolysis, including mechanisms of reovirus resistance; (iv) the potential benefits of genetically-modified reovirus platforms; (v) reovirus scheduling to maximize virus delivery and efficacy including the best route of virus administration; and (vi) combinatorial approaches that are designed to boost both direct oncolysis and anti-tumour immune responses. PAMPs: pathogen-associated molecular patterns; DAMPs: damage-associated molecular patterns; GM-CSF: granulocyte-macrophage colony stimulating factor. Figure created using Biorender.com.