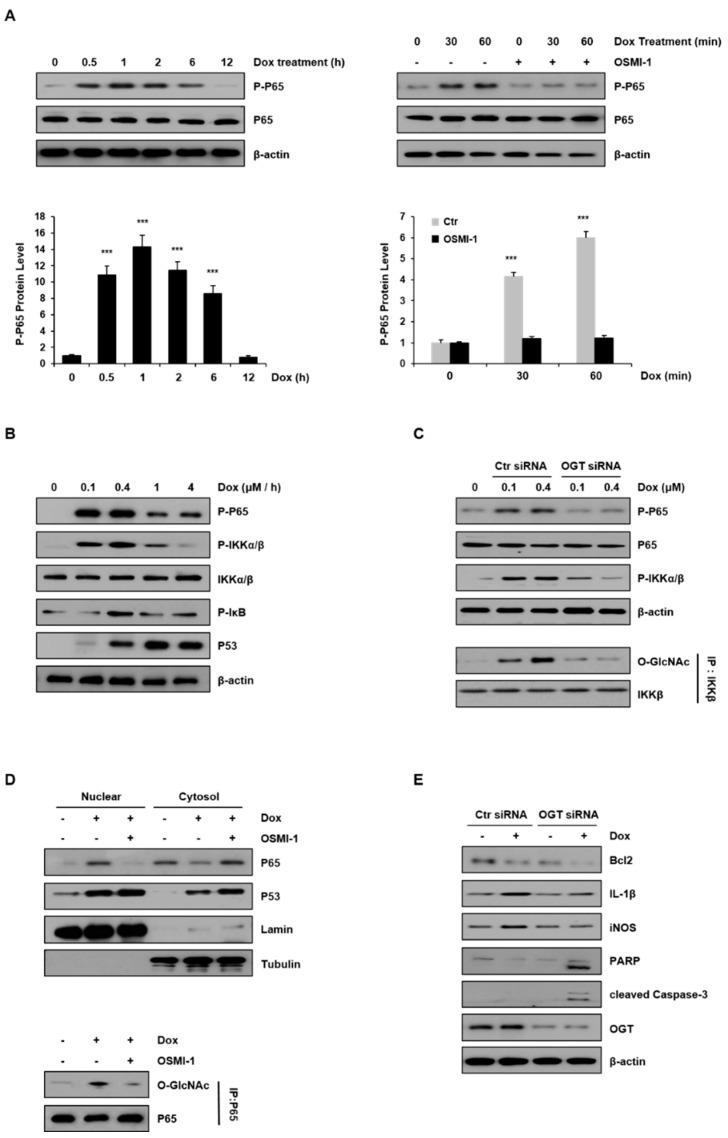
Figure 4.
Inflammatory NF-κB signaling was modulated by DOX and OSMI-1. (A) Levels of phospho-p65 in HepG2 cells treated with DOX (0.4 μM) were monitored by Western blot for up to 12 h (left). HepG2 cells were treated with DOX (0.4 μM) for up to 60 min in the absence or presence of OSMI-1 (20 μM) and levels of phospho-p65 were analyzed (right). The lower two graphs show the relative amount of these proteins as fold increase. Data represent three independent experiments. *** p < 0.005 compared with the control group. (B) HepG2 cells treated with DOX (0 to 4 μM) for 1 h were analyzed by Western blot using antibodies against phospho-p65, phospho-IKKα/β, phospho-IκB, and p53. (C) After transfection with control or OGT siRNAs, HepG2 cells were treated with DOX (0.1 and 0.4 μM) for 1 h. Levels of phospho-p65 and phospho-IKKα/β in HepG2 cells were analyzed by Western blot. Immunoprecipitation analysis using the IKKβ antibody was performed (lower panel) followed by immunoblotting using the GlcNAc antibody. Immunoprecipitation analysis was performed using whole-cell lysate, and the GlcNAc levels were assessed using the same amount of precipitates. (D) OSMI-1 (20 μM) pretreated with or without HepG2 cells were treated with DOX (0.4 μM) for 1 h. The levels of p65 and p53 in the nuclear and the cytoplasmic fractions were analyzed by Western blot. LaminB and tubulin were used as loading controls for the nuclear and cytoplasmic proteins, respectively. Nuclear fractions from the cell lysates were subjected to immunoprecipitation using anti-p65 antibodies, followed by Western blot analysis with anti-O-GlcNAc antibodies (right). (E) After transfection with scramble-siRNA or OGT siRNA, HepG2 cells were treated without or with DOX (0.4 μM) for 15 h. Total lysates were subjected to Western blot analysis using the indicated Abs, the uncropped western blot figures in Figure S6.