Figure 1.
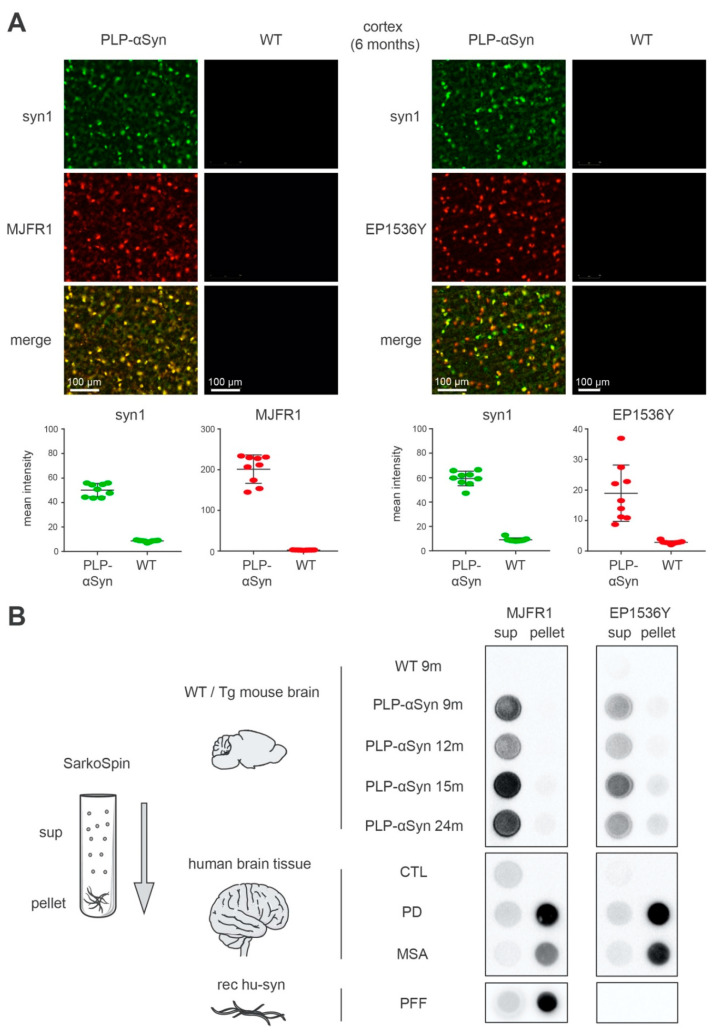
The S129-phosphorylated α-syn species found in the brain of PLP-αSyn mice are distinct from the amyloid forms extracted from Parkinson’s disease (PD) and multiple system atrophy (MSA) brains and from recombinant preformed fibrils (PFFs). (A) Immunofluorescence staining of 6 months-old PLP-αSyn mouse cortical sections, representative of 3 independent experiments. Total α-syn (syn1, green, top panels) shows a complete colocalization with human α-syn (MJFR1, red, mid-left panels) and partial colocalization with pS129 phosphorylated α-syn (EP1536Y, red, mid-right panels). The 4 lower panel quantifications depict the mean field intensity values of 9 cortical/striatal fields of views at 20× from the sections shown above and confirm the massive overexpression of α-syn in PLP-αSyn mouse brains compared to wild-type (WT). (B) Biochemical aggregation analysis of the different species of α-syn found in WT or PLP-αSyn mouse brains (top), control, PD and MSA human subject brains (middle) or a preparation of recombinant human α-syn PFF (bottom). Pooled 9 to 24 months-old mice (n = 3) or human (n = 3) brain homogenates and PFF samples were subjected to SarkoSpin procedure consisting of a sarkosyl solubilization at 37 °C with nuclease under shaking followed by an ultracentrifugation on sucrose cushion. The contents in human α-syn (MJFR1, left panel) and pS129-α-syn (EP1536Y, right panel) of SarkoSpin supernatant and pellet fractions were assessed by filter trap followed by immunolabelling with the respective antibodies. Pictures are representative of n = 3 independent Sarkospin procedures quantified in Supplementary Figure S1.