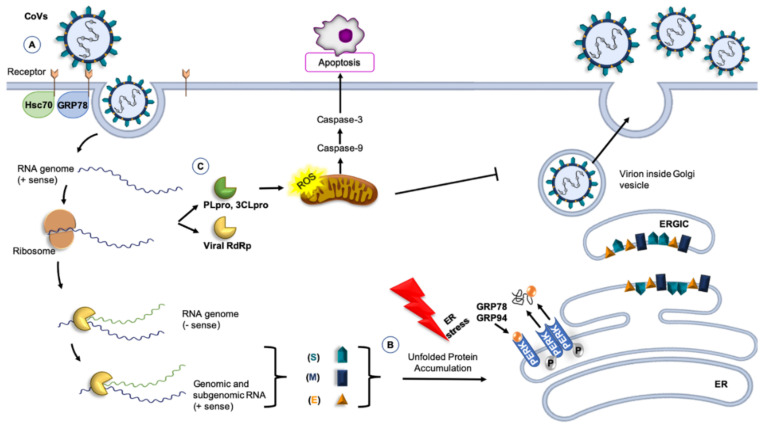
Figure 1.
CoVs and molecular chaperones. (A) GRP78 or 74-kDa heat shock cognate protein 70 (Hsc70) can be part of the receptor complex recognized by the CoVs and can modulate virus entry [82,83,84]. (B) Molecular chaperones, such as GRP78 and GRP94, participate in the folding of CoV proteins, counteracting the effect of the stress of the host cell caused by viral infection. Accumulation of unfolded proteins induces the transcription of GRP78 and GRP94. In the endoplasmic reticulum (ER) lumen, the release of GRP78 and GRP94 from PERK are protective, inducing the unfolded protein response and controlling proteins folding. Under these conditions, ER homeostasis is restored [85]. (C) Non-structural proteins, such as 3C-like serine protease (3CLpro), induce apoptosis thought caspase’s pathways, causing a significant increase in reactive oxygen species (ROS) [86]. Other abbreviations: RBD, receptor-binding domain (of the S protein); PLpro, papain-like cysteine protease; RdRp, RNA-dependent RNA polymerase; PERK, PKR-like endoplasmic reticulum kinase; ERGIC, Endoplasmic reticulum-Golgi intermediate compartment.