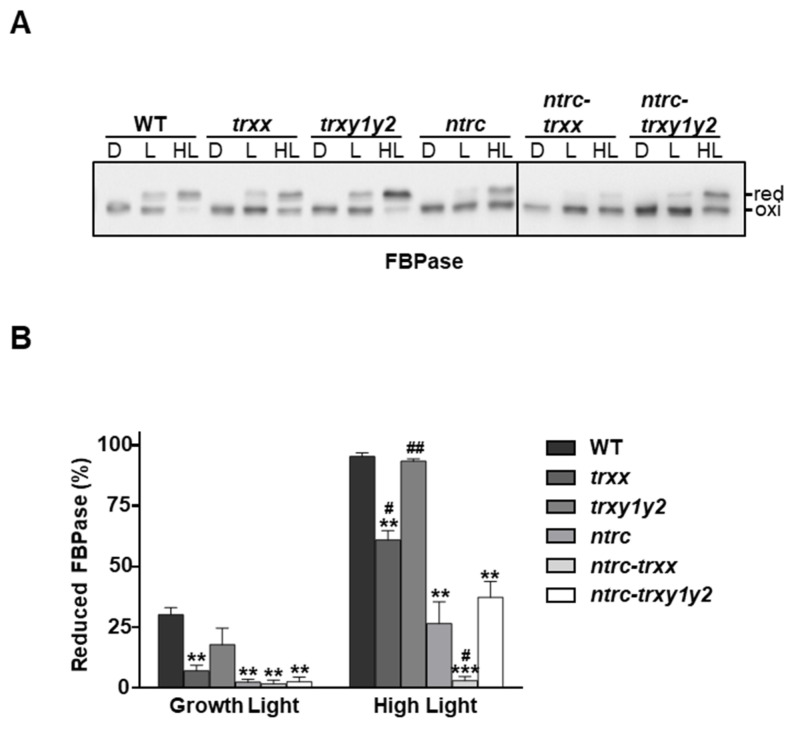
Figure 4.
In vivo redox state of FBPase in mutant plants devoid of Trxs y. Plants of the wild type and mutant lines, as indicated, were grown under long day conditions for 4 weeks. Whole rosettes were harvested at the end of the period of darkness (D) and then illuminated for 30 min with light intensities of 125 μE m−2 s−1 (growth light, L) or 450 μE m−2 s−1 (high light, HL). (A) The in vivo redox state of FBPase was determined by alkylation using the thiol-labelling agent iodoacetamide (IAA). Protein extracts were subjected to SDS–PAGE under non-reducing conditions, transferred onto nitrocellulose filter and probed with an anti-FBPase antibody. (B) Band intensities were quantified (GelAnalyzer), and the percentage of reduction was calculated as the ratio between the reduced form and the sum of oxidized and reduced forms. Each value is the mean of three independent experiments ± standard error (SE). Statistical significance compared with the wild type (asterisks) or the ntrc mutant (hashes) is indicated (#, p < 0.05; ** and ##, p < 0.01; ***, p < 0.001, Student’s t test); red, reduced; oxi, oxidized.