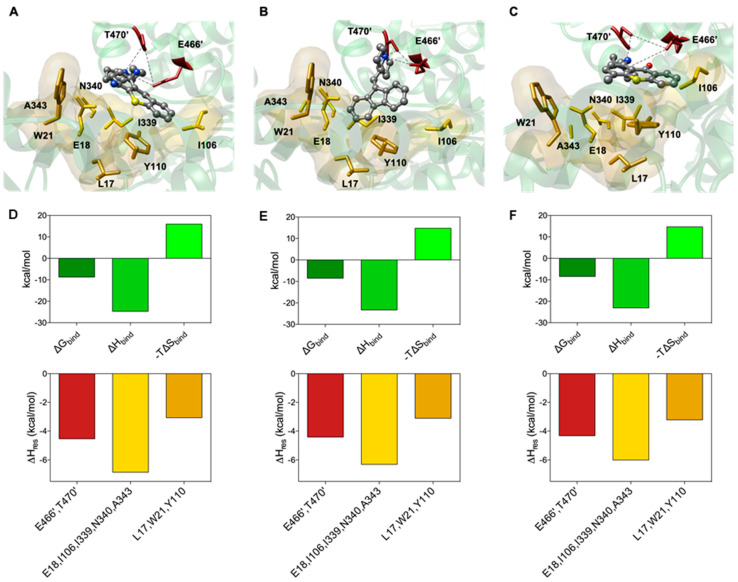
Figure 5.
(top panel) Details of compounds 14 (A), 17 (B) and lucanthone (C) in the binding pocket of TryR. Compounds are shown as atom-colored sticks-and-balls (C, grey, N, blue, O, red). The side chains of the mainly interacting TryR residues are depicted as colored sticks and labeled as following: E466′ and T470′, firebrick; E18, W21, I339, N340 and A343, gold; L17, I106 and Y110, goldenrod. The hydrophobic pockets are also highlighted by their transparent van der Waals surface. Hydrogen atoms, water molecules, ions and counterions are omitted for clarity. (bottom panel) Calculated free energy of binding (ΔGbind, forest green), and enthalpic (ΔHbind, lime green) and entropic (−TΔSbind, chartreuse) components (upper row) and PRBFED of the main involved amino acids (bottom row) of TryR in complex with 14 (D), 17 (E) and lucanthone (F).