Figure 2.
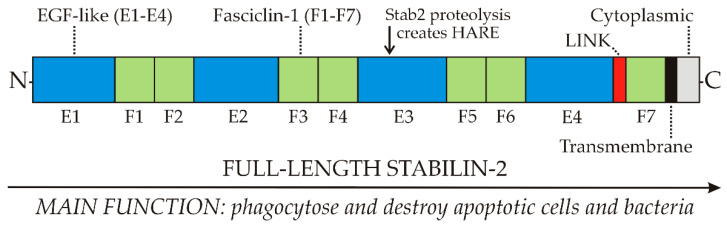
Human Stab2 domain organization. Full-length Stab2 is a type I membrane protein [17] of 2551 aa with a large N-terminal extracellular domain, a 92 aa HA-binding Link module (red), a 22 aa transmembrane domain (TMD, black), and a 72 aa C-terminal cytoplasmic domain (CD, gray). The ectodomain contains 7 Fasciclin-1 domains (F1–F7; green) and 4 larger EGF-like domains (blue; E1–E4) spanning the ectodomain length. Multiple binding sites for phosphatidylserine (PS) and for αMβ2 and αvβ5 integrins are within the E1-E4 and F1–F7 domains, respectively. PS binding sites enable receptor recognition of dying apoptotic cells [18] and integrin-binding sites enable receptor recognition of lymphocytes [19] in coordination with PS interactions [20]. A proteolytic cleavage site (arrow) that generates the 190 kDa HARE isoform at Ser1136 [21] is within Fasciclin-1 domain 4 (F4). This constitutive cleavage process gives two half-receptors: a soluble N-terminal half, whose fate is unknown, and the membrane-bound C-terminal half that is HARE (Section 5).