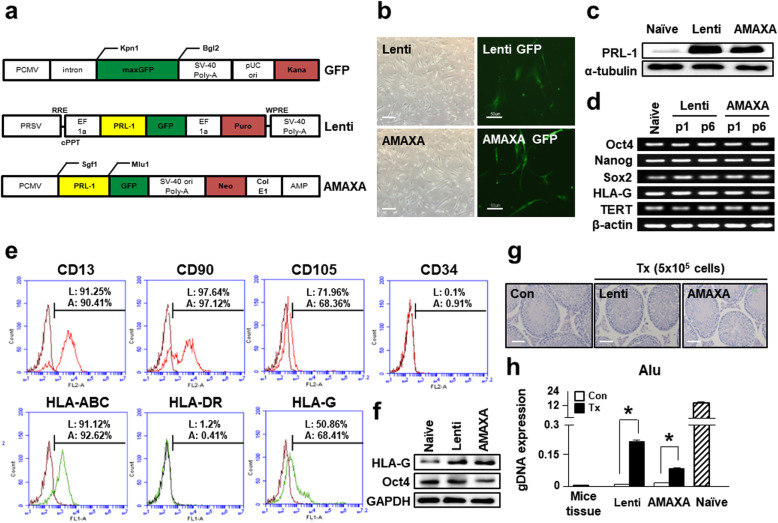
Fig. 1.
Generation of stable PD-MSCs with PRL-1 (PD-MSCsPRL-1, PRL-1+) using lentiviral and nonviral AMAXA systems. a GFP, lentiviral, and nonviral AMAXA plasmid vector map. b Morphology of PD-MSCsPRL-1 using lentiviral and nonviral AMAXA systems. Scale bars = 100 μm. The expression of GFP in PD-MSCs after each transfection system. Scale bars = 50 μm. c Western blotting of PRL-1 expression in naïve and PD-MSCsPRL-1. d RT-PCR analysis of stemness markers in naïve and PD-MSCsPRL-1 depending on passage. e FACS analysis of surface markers related to hematopoietic, nonhematopoietic, and HLA family members in PD-MSCsPRL-1. f Western blotting of Oct4 and HLA-G expression in naïve and PD-MSCsPRL-1. g H&E staining of normal and PD-MSCPRL-1-transplanted NOD/SCID mouse testes at 14 weeks (lenti; n = 2, AMAXA; n = 2). Scale bars = 50 μm. h Engraftment of PD-MSCsPRL-1 into mouse testes posttransplantation. Mouse tissue was used as a negative control. Human PD-MSCs were used as a positive control. Data from each group are expressed as the mean ± SD. *p < 0.05 versus the Con group. Con, control; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GFP, green fluorescent protein; PRL-1, phosphatase of regenerating liver-1; TERT, telomerase reverse transcriptase