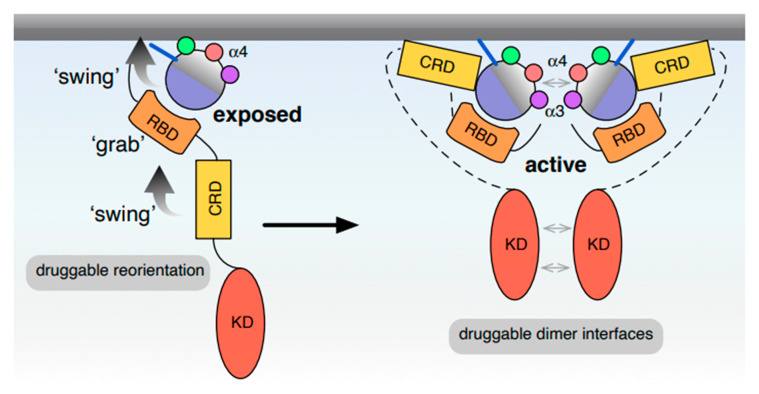
Figure 4.
New model for Ras reorientation and RAF-dependent nanoclustering. We propose a ‘grab-and-swing’ model, where reorientation dynamics contribute to the full membrane engagement of Ras-bound binding partners such as the effector RAF (left). The rearrangement of the whole membrane bound complex is then further stabilized by at least transient dimer interfaces of Ras. Note that high-affinity, rigid Ras dimer formation could impair the activity of the whole complex, while allosteric coupling between the Ras-engaged N-terminus of RAF and its C-terminus reinforces dimerization of the kinase domain, which is required for RAF activation.