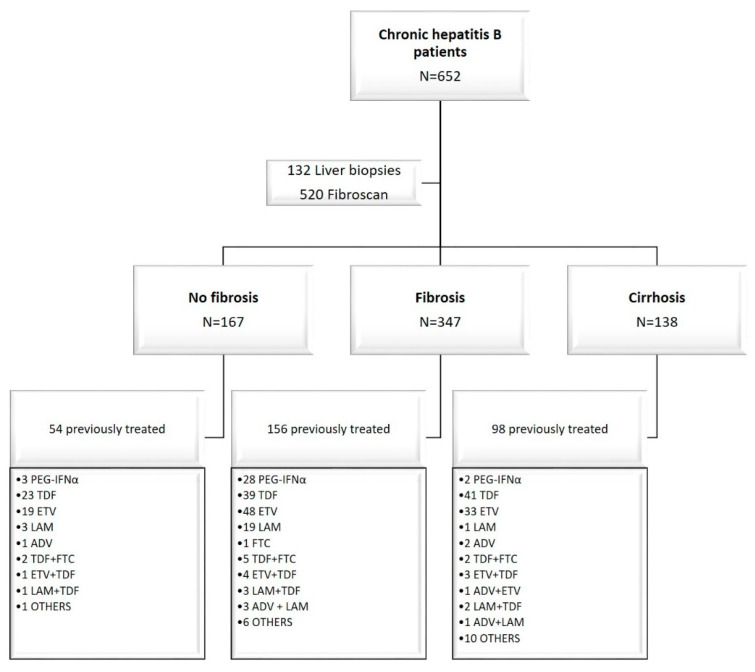
Figure 1.
Disposition of enrolled patients. The liver assessment was performed at the time of enrollment in the study, and the treatment history was provided simultaneously. The median of treatment before the liver fibrosis assessment was 3 months. PEG-IFNα, pegylated interferon alfa; TDF, tenofovir; ETV, entecavir; LAM, lamivudine; ADV, adefovir; FTC, emtricitabine; OTHERS: ADV + ETV + LAM + TDF (2), PEG-IFNα + TDF (2), PEG-IFNα + ETV (2), ADV + LAM + TDF (2), vidarabine (1), famciclovir (1), PEG-IFNα + LAM + TDF (2), anti HBsAg monoclonal antibodies (2), anti HBsAg monoclonal antibodies + TDF (2), anti HBsAg monoclonal antibodies + ETV (1).