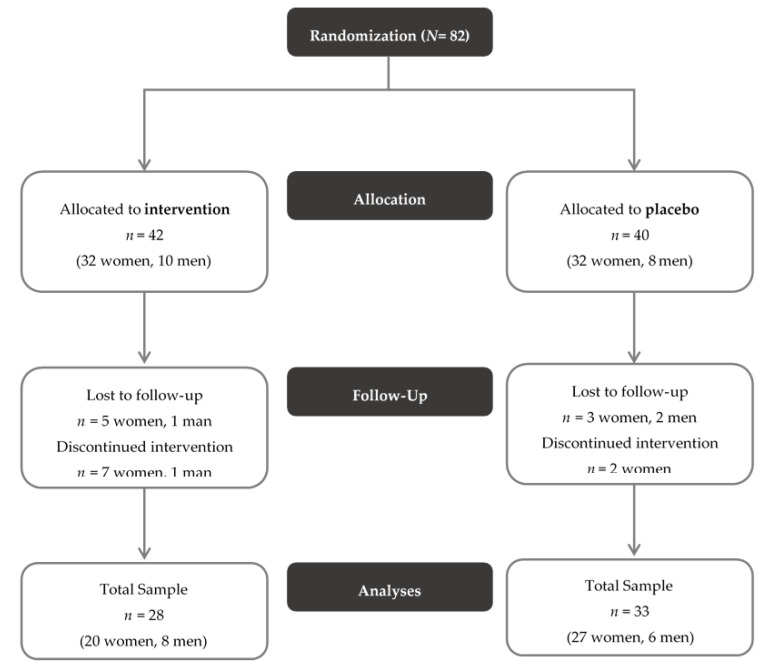
Figure 1.
CONSORT Flow Diagram of the PROVIT study. Exclusion criteria consisted of acute suicidality, lack of consent, pregnancy or breastfeeding, severe active drug dependence (i.e., alcohol, benzodiazepines, morphine), other currently active severe cerebral organic diseases (e.g., epilepsy, brain tumor), severe skull- brain trauma/brain surgery in the past, known florid tumor disease, congenital/infantile mental disability, moderate/severe dementia, severe florid autoimmune diseases or current immunosuppression (e.g., lupus erythematosus, HIV, multiple sclerosis), antibiotic therapy within the last month, chronic laxative abuse, acute infectious diarrheal disease, regular intake of butyrate-containing or probiotic supplements in the last year, intake of (other) probiotics and prebiotics or butyrate preparations during the entire trial or within the last month and intake of antibiotics or prebiotics during the entire trial or within the last month.