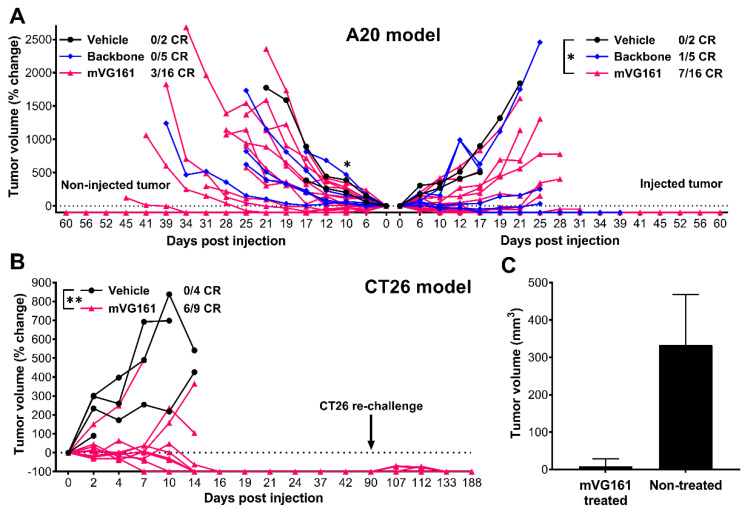
Figure 7.
In vivo efficacy of a murine version of VG161 (mVG161) following intratumoral inoculation. (A) A20 cells were subcutaneously implanted into immunocompetent BALB/C mice in both sides of lower flanks (5 × 106 A20 cells per flank). 5 × 106 PFU/mouse/day of either mVG161 or VG160 backbone virus (version of VG161 without payload) was injected once per day for 5 consecutive days into tumors on one side only. 16 mice were treated with mVG161, 5 mice were treated with VG160, and 2 mice were treated with vehicle (PBS) control. (B) Thirteen immunocompetent BALB/C mice were subcutaneously implanted with 1 × 106 CT26 cells/mouse into the lower flanks, with 9 animals randomly assigned to the mVG161 treatment group (5 × 106 PFU/mouse injected 5 times) and another 4 animals to the vehicle (PBS) control group. At 90 days post injection, the surviving 6 mice in the mVG161-treated group were re-implanted with 1 × 106 CT26 cells in the same location. (C) Tumor sizes 7 days after CT26 re-challenge in mVG161-treated animals compared to age-matched (28 weeks old) control mice that were not treated with mVG161. CR = complete response. * p < 0.05, ** p < 0.01. p values were computed using unpaired t-test. Error bars indicate SD.