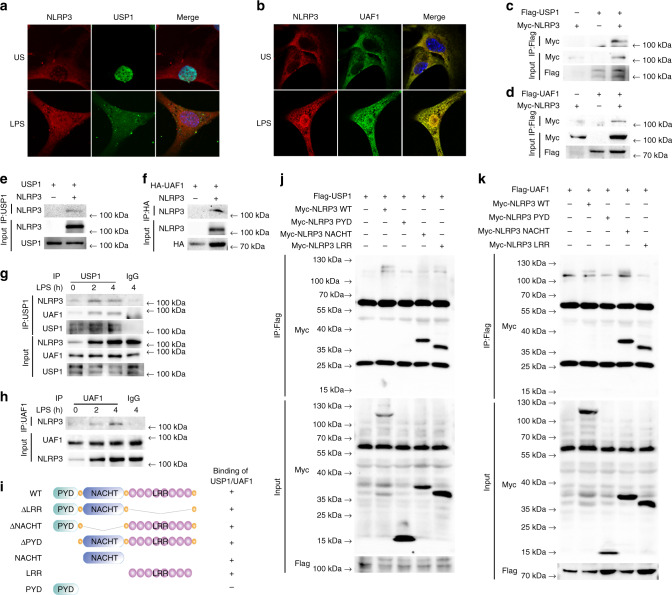
Fig. 5. UAF1/USP1 interacts with NLRP3.
a, b Confocal microscopy analysis of colocalization of NLRP3 with USP1 or UAF1. MEFs transfected with Myc-NLRP3, GFP-USP1 or Flag-UAF1 were stimulated with LPS for 2 h, then fixed and incubated with a secondary antibody conjugated to Alexa Fluor 637 or Alexa Fluor 488. Scale bar, 10 µm. c, d Extracts from HEK293T cells transiently transfected with Flag-USP1 and Myc-NLRP3 (c), and Flag-UAF1 and Myc-NLRP3 (d) were subjected to IP with anti-Flag, followed by western blot analysis with anti-Myc and anti-Flag. e PCMV6-USP1 and Myc-tagged NLRP3 were obtained by in vitro transcription and translation. Interaction between USP1 and NLRP3 was analyzed by mixing USP1 and NLRP3 together, followed by IP with USP1 antibody and immunoblot analysis with NLRP3 and USP1 antibody. f HA-tagged UAF1 and Myc-tagged NLRP3 were obtained by in vitro transcription and translation. Interaction between UAF1 and NLRP3 was assayed by mixing UAF1 and NLRP3 together, followed by IP with HA antibody and immunoblot analysis with NLRP3 and HA antibody. g, h Extracts of peritoneal macrophages stimulated with LPS for the indicated time periods were subjected to immunoprecipitation with anti-USP1 (g) and anti-UAF1 (h) followed by western blot analysis with indicated antibody. Proteins from a whole-cell lysate were used as positive control. i Schematic diagram of NLRP3 and its truncation mutants. j, k Myc-tagged NLRP3 or its mutants along with Flag-USP1 (j) or Flag-UAF1 (k) were individually transfected into HEK293T cells. The cell lysates were immunoprecipitated with Flag antibody and then immunoblotted with the indicated antibodies. US, unstimulated. Similar results were obtained from three independent experiments.