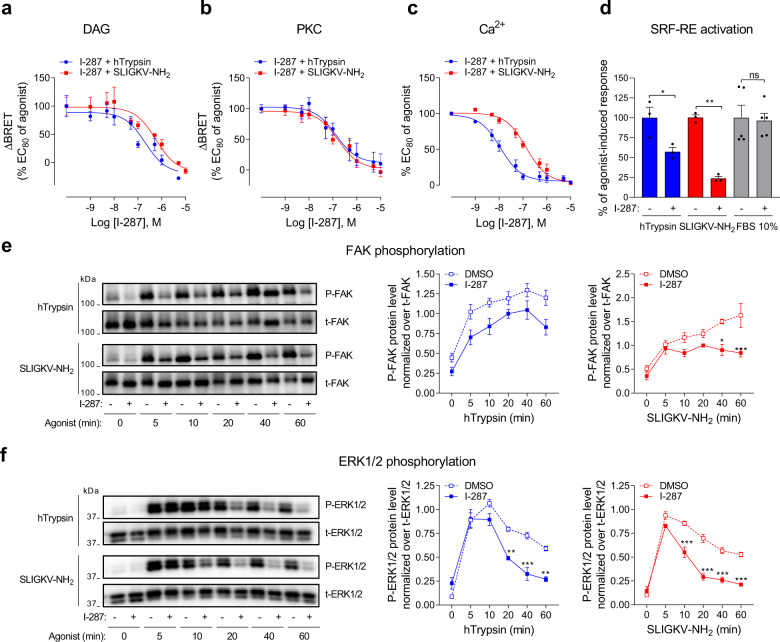
Fig. 5. I-287 inhibits PAR2-mediated activation of DAG/Ca2+/PKC and RhoA/SRF-RE, as well as FAK and ERK1/2 signaling pathways.
a, b Impact of increasing concentrations of I-287 (15 min) on DAG production (a) and PKC activation (b) induced after 1 (DAG) or 5 (PKC) min stimulation with an EC80 concentration of hTrypsin or SLIGKV-NH2 in HEK293 cells co-expressing hPAR2 and the indicated unimolecular BRET2-based biosensors. Results are expressed as ΔBRET in % of the response induced by EC80 of respective agonists in the absence of I-287 (mean ± SEM; n = 4–5). c Impact of increasing concentrations of I-287 (30 min) on intracellular Ca2+ mobilization induced by an EC80 concentration of hTrypsin or SLIGKV-NH2 in HEK293 cells endogenously expressing hPAR2. Results are expressed as % of the response induced by respective agonists in the absence of I-287 (mean ± SEM; n = 3-4). d Impact of I-287 (10 µM, 30 min) on hPAR2-promoted SRF-RE reporter gene activation induced after 6 h stimulation with hTrypsin (10 U/mL) or SLIGKV-NH2 (100 µM) in HEK293 cells expressing hPAR2. FBS (10%) was used as control. Results are expressed as % of the response induced by respective agonists in the absence of I-287 (mean ± SEM; n = 3–5; unpaired t-test: *p < 0.05 and **p < 0.01 compared to respective control cells, ns: nonsignificant). e, f Kinetics of FAK and ERK1/2 phosphorylation in HEK293 cells expressing hPAR2 and pretreated with DMSO or I-287 (10 µM, 30 min) before stimulation with hTrypsin (1 U/mL) or SLIGKV-NH2 (100 µM) at the indicated times. Representative immunoblots of FAK and ERK1/2 phosphorylation are shown. Western blots were quantified and expressed as the ratio of phosphorylated protein level (P-FAK or P-ERK1/2) normalized over total protein (t-FAK or t-ERK1/2; mean ± SEM; n = 3–5; two-way ANOVA followed by Tukey’s post hoc test: *p < 0.05, **p < 0.01, and ***p < 0.001 compared to DMSO-treated cells at the respective time).