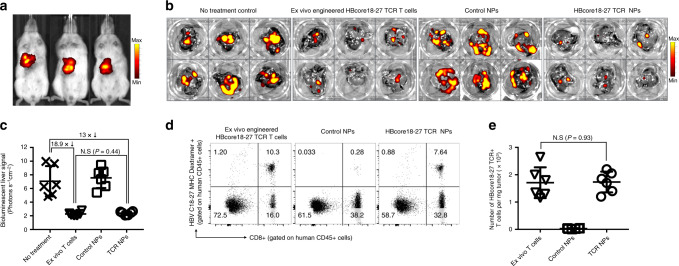
Fig. 8. In situ programming of HBV-specific T cells using nanoparticles loaded with TCR transgenes.
a We established a mouse xenograft tumor model of HBV-induced HCC. HepG2 cells stably transduced with HBcAg and luciferase were surgically injected into the liver of NSG mice reconstituted with human T cells. Three weeks post-implantation, HepG2 tumors were visualized by in vivo bioluminescent imaging and assigned to nanoparticle (6 weekly doses of 50 μg mRNA encoding the HBcore18-17 TCR) or T-cell treatment (5 × 106 T cells transduced ex vivo with lentiviral vectors encoding the HBcore18-17 TCR) groups. b, c Quantification of bioluminescent liver signal 6 weeks after treatment start. Shown are mean values ± SD; two tailed unpaired Student’s t-test. N = 5 biologically independent samples. d Multicolor flow cytometry of cells recovered from the liver 18 days after treatment start. Adoptively transferred or in situ-programmed HBcore18-27 TCR+ T cells were identified by positive labeling for CD45, CD8, and MHC Pentamer. Absolute numbers are shown in e. Total cell counts of viable (trypan blue-negative) cells were multiplied by the percentage that was HBcore18–27 TCR+, CD8+, and CD45+. Shown are mean values ± SD. Pairwise differences in absolute numbers of T cells between the groups were statistically analyzed by two tailed unpaired Student’s t-test. P < 0.05 was considered significant. N.s. nonsignificant. N = 5 biologically independent samples.