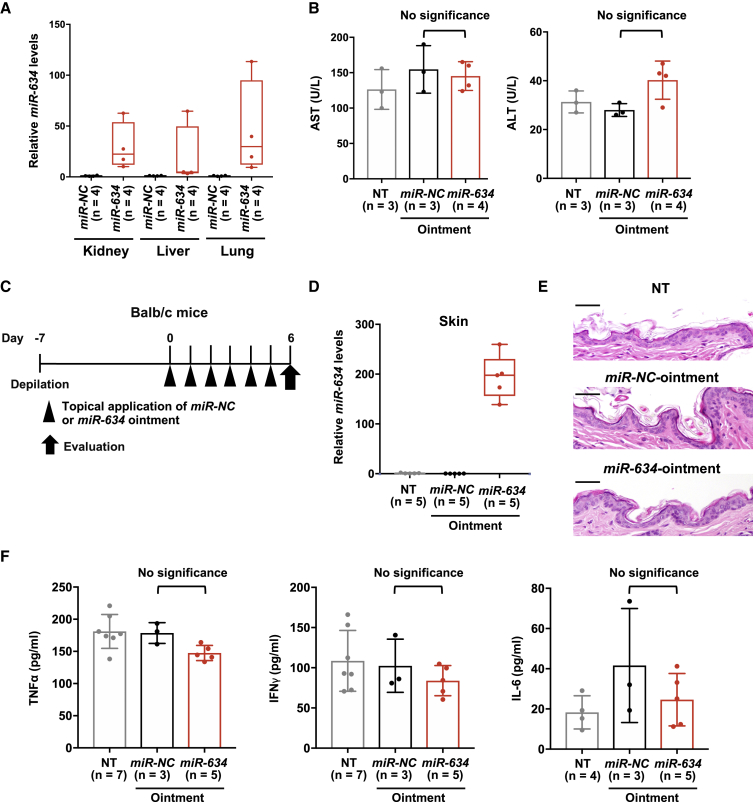
Figure 2.
Safety of the Topical Application of miR-634 Ointment in Mice
(A) qRT-PCR expression analysis of miR-634 in the kidneys, liver, and lungs. miR-634 expression levels in normal tissues from mice treated with miR-NC ointment (n = 4) or miR-634 ointment (n = 4) were measured by qRT-PCR, and the results are presented in the boxplot. (B) Plasma AST (left) and ALT (right) levels in mice treated with miR-NC ointment (n = 3) or miR-634 ointment (n = 4) at 6 days after initial treatment and in control mice (no treatment [NT]) (n = 3). Error bars indicate the SD. Data are presented as mean ± SD. p values were calculated using the one-way ANOVA (p = 0.893 for AST, p = 0.065 for ALT). (C) Experimental schedule for the application of miR-634 ointment in BALB/c mice. (D) miR-634 expression analysis in skin tissues by qRT-PCR. miR-634 levels in skin tissues from mice treated with miR-NC ointment (n = 5) or miR-634 ointment (n = 5) and from control mice (NT) (n = 5) were measured by qRT-PCR, and the results are presented in the boxplot. (E) H&E staining of skin tissues from mice treated with miR-NC ointment or miR-634 ointment and from control mice (NT). Scale bars, 50 μm. (F) Serum TNF-α, IFN-γ, and IL-6 levels in mice treated with miR-NC ointment or miR-634 ointment at 6 days after initial application and in in control mice (NT). Error bars indicate the SD. Data are presented as mean ± SD. p values were calculated using the one-way ANOVA (p = 0.145 for TNF-α, p = 0.713 for IFN-γ, and p = 0.381 for IL-6).