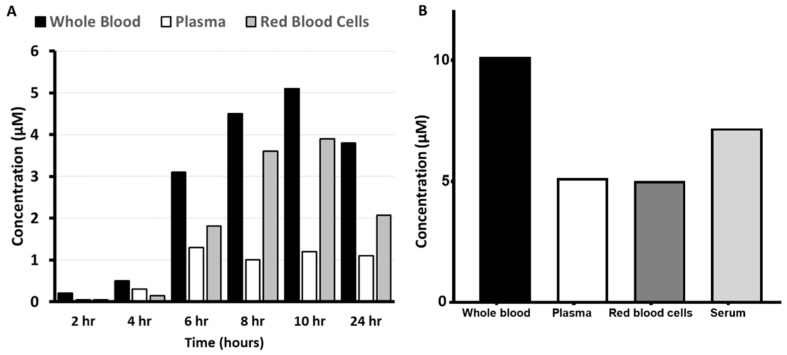
Figure 8.
Plasma pharmacokinetics of sunitinib (A) and sorafenib (B). Samples for sunitinib were obtained over the first 24 h after drug ingestion from one single naïve patient with an advanced solid tumor, refractory to standard treatment, receiving oral sunitinib at 700 mg per week. Samples for sorafenib were obtained at steady state conditions (30 days after initiation of treatment) from one representative patient receiving 400 mg sorafenib twice daily.