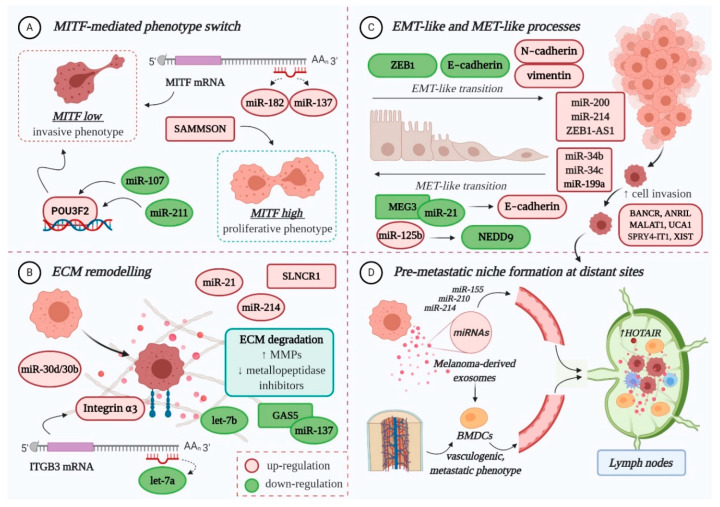
Figure 1.
The role of non-coding RNAs in melanoma invasion and metastasis: (A) Both miRNAs and lncRNAs modulate the expression of MITF-M, directly or indirectly, therefore influencing the switch towards an invasive or proliferative phenotype; (B) The deregulated expression of ncRNAs facilitates ECM remodelling, by targeting integrins, increasing matrix metalloproteinases (MMPs) and diminishing the metallopeptidase inhibitors (TIMPs), therefore promoting ECM degradation and decreasing tumour cell-matrix adhesion; (C) Multiple miRNAs and lncRNAs favour the EMT-like process of melanoma cells, which enhances their migratory capacity, mainly by affecting the expression of specific adhesion molecules. Some ncRNAs also promote the MET-like process of malignant melanocytes, which leads to enhanced proliferation and colony formation, thus favouring metastasis; (D) The formation of a pre-metastatic niche at distant sites is mainly influenced by melanoma-derived exosomes, enriched in miRNA cargo (e.g., miR-155, miR-210, miR-214). In sentinel lymph nodes, they instigate microenvironmental changes that facilitate the recruitment, trapping and growth of circulating tumour cells. They also prime bone-marrow-derived cells (BMDCs), which achieve a vasculogenic, metastatic phenotype, and facilitate their recruitment to distant sites, where BMDCs will contribute to the formation of a pre-metastatic niche. LncRNA HOTAIR is enriched in lymph node metastases compared to primary tumours, which may suggest a role in the pre-metastatic niche formation. POU3F2: POU-domain class 3 transcription factor 2; NEDD9: Neural precursor cell expressed developmentally down-regulated protein 9; ZEB1: zinc finger E-box-binding homeobox 1. Colour code: red for upregulation and green for downregulation. Figure created with BioRender.com.