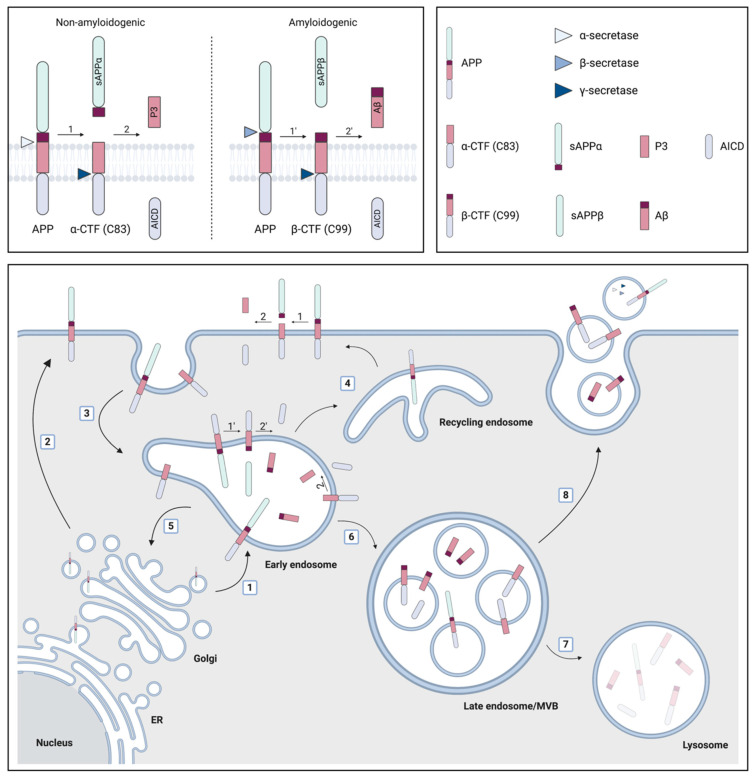
Figure 1.
The role of multivesicular bodies (MVBs) and extracellular vesicles (EVs) in amyloid precursor protein (APP) processing. APP is synthesized in the endoplasmic reticulum (ER) and subsequently transported through the Golgi compartment to early endosomes (1) or the plasma membrane (2), where it can be cleaved by α-secretase in the non-amyloidogenic pathway. This cleavage leads to the formation of soluble APP α (sAPPα) and the α-C-terminal fragment (α-CTF or C83). Alternatively, APP can be re-internalized in endosomes (3) where the amyloidogenic cleavage by β-secretase preferentially occurs. Thereby, sAPPβ and β-CTF (C99) are generated. The formation of the APP intracellular domain (AICD) and either the P3 fragment or the amyloid β (Aβ) peptide from α- and β-CTF, respectively, requires cleavage by the γ-secretase complex. Early endosomes recycle to the plasma membrane (4), undergo retrograde transport to the trans-Golgi network (5) or mature into late endosomes/MVBs (6). The latter either fuse with lysosomes (7) or with the plasma membrane (8), resulting in the extracellular release of their intraluminal vesicles (ILVs) as EVs. Several products of the APP processing pathway have been localized to MVBs (i.e., APP itself; α- and β- CTF; AICD; Aβ) and EVs (i.e., APP itself; α- and β- CTF; Aβ; α-, β- and components of the γ-secretase complex), whereby Aβ can be localized both on the inside and on the surface of EVs. Figure created with BioRender.