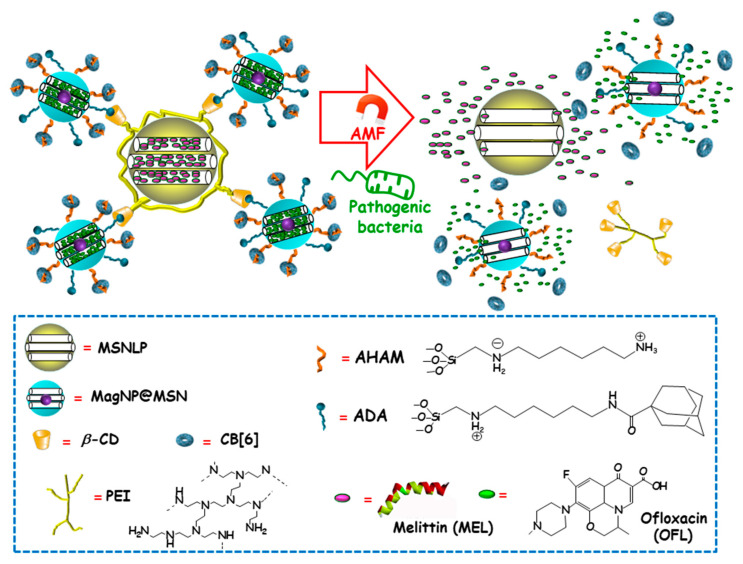
Figure 11.
Schematic representation of the operating mechanism of dual drug-delivery and pathogen/heating/alternating magnetic field (AMF)-responsive antimicrobial nanosystems formed by the supramolecular co-assembly of host MSNs (H), guest MSNs (G). H consisted of large-pore MSNs (MSNLP) loaded with melittin (MEL) and capped by β-cyclodextrin (β-CD)-modified polyethylenimine (PEI). G consisted of MnFe2O4@CoFe2O4 magnetic NPs coated with a mesoporous silica layer (MagNP@MSN), loaded with ofloxacin (OFL) and decorated with both adamantine (ADA) (to interact with β-CD on the surface of H) and N-(6-N-aminohexyl)aminomethyl triethoxysilane (AHAM) (to interact with cucurbit[6]uril (CB[6])) for efficient pore capping. Adapted from ref. [101]. Dual antimicrobial drug release is triggered by the presence of pathogenic cells and the application of an alternating magnetic field (AMF).