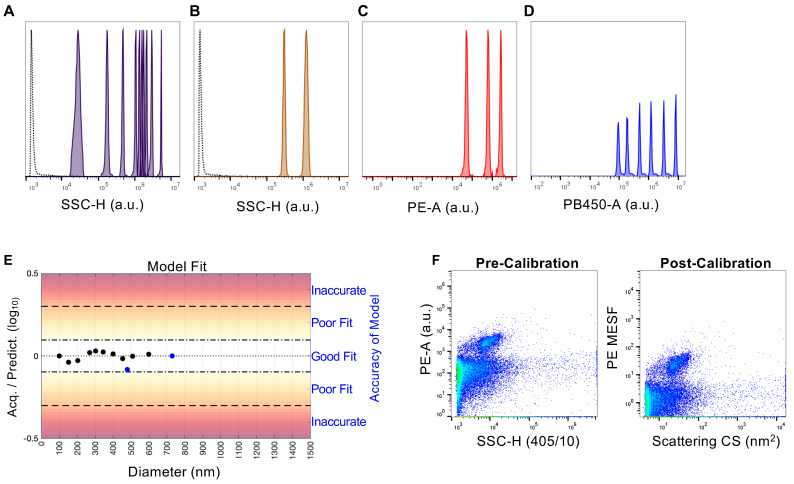
Figure 2.
Standardization of flow virometry data with reference beads for light scatter and fluorescence calibration using FCMPASS software. (A) NIST-traceable polystyrene (100, 152, 203, 269, 303, 345, 401, 453, 508, 600 nm) and (B) silica calibration beads (480, 730 nm) used to perform side scatter calibration. Bead populations are displayed from left to right in order of increasing diameter (i.e., higher arbitrary unit, a.u.). The empty dotted histogram denotes instrument background noise from PBS. (C) Quantibrite PE beads and (D) Rainbow calibration particles used for fluorescence calibration, as detected with the same gains used for virus sample acquisition on the flow cytometer. Bead populations are listed from left to right in order of increasing fluorescence (higher a.u.). (E) FCMPASS output assessing the fit of our data generated on the CytoFLEX cytometer for scatter modeling by comparing the predicted versus acquired scatter values for the sizing beads of differing diameters (black and blue data points represent polystyrene and silica beads, respectively). (F) Representative plots comparing uncalibrated data expressed in arbitrary units of fluorescence intensity to calibrated data in standard units (MESF, nm2), using CD14+ HIV stained with an anti-CD14-PE antibody as representative data. Fluorescence and light scattering calibrations were performed on all datasets used in this manuscript, with consistent calibration values generated across all datasets.