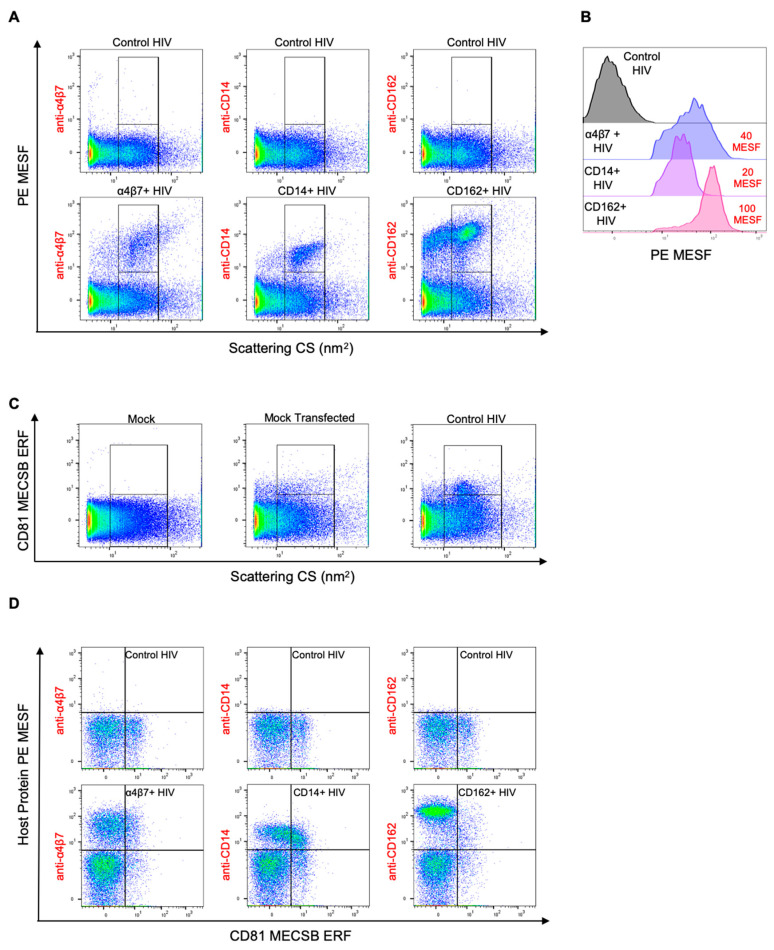
Figure 4.
Phenotypic analysis of cellular proteins on the surface of HIV pseudoviruses. (A) Single staining of cellular proteins on virions with incorporated host proteins (α4β7+ HIV, CD14+ HIV, CD162+ HIV) using PE-conjugated antibodies specific for the respective incorporated cellular proteins. Lower gates are set for the side scattering population of control viruses. The lower gate spans 10–60 nm2 on the x-axis and has an upper limit of 10 PE MESF on the y-axis. Upper gates display positive host protein staining as determined using the control virus (in top row) and extend above the background fluorescence (>10 PE MESF on the y-axis). (B) A comparison of cellular protein levels on each of the transfected virus populations, as identified from the upper gates in (A), with the median PE MESF value of each population shown in red (top right). The control virus (grey) was identified using the lower gate from (A). (C) CD81 tetraspanin staining of cell culture media, mock-transfected cell supernatants, and control HIV. Transfection of HEK293 cells with a mock vector induced the release of CD81-positive non-virus particles, as identified by the upper gate. The lower gate was set on events generated by acquiring cell culture media alone. (D) Dual labeling for transfected cellular proteins and tetraspanin in HIV viruses using PE-conjugated antibodies against cellular proteins (α4β7, CD14, CD162) and a BV421-conjugated anti-CD81 antibody. BV421 is expressed in equivalent reference fluorophore (ERF) units of molecules of equivalent cascade blue (MECSB). The events shown are representative of the total virus populations from both gates in (A). Gating controls are shown in Figure S6. The results are representative of three technical replicates.