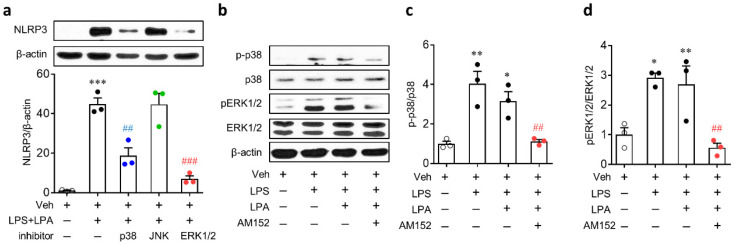
Figure 8.
Activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) is involved in LPA1-mediated NLRP3 upregulation in LPS-primed BMDMs, followed by LPA exposure. Cells were primed with LPS (500 ng/mL) for 4 h, followed by an exposure to LPA (1 µM) for 1 h. Protein expression levels of NLRP3 and phosphorylation of p38 and ERK1/2 were determined by Western blots. (a) Effects of MAPK inhibitors on NLRP3 upregulation in LPS-primed BMDMs based on Western blot analysis. Representative blots and quantification data are shown. Cells were treated with each inhibitor for 30 min prior to LPS priming. n = 3 per group. *** p < 0.001 versus control BMDMs (Veh). ## p < 0.01 and ### p < 0.001 versus stimulated BMDMs (LPS + LPA). (b-d) Effects of LPA1 antagonist on activation of p38 or ERK1/2 in LPS-primed BMDMs determined by Western blot analysis. Representative Western blots of phosphorylated p38 (p-p38), total p38 (p38), phosphorylated ERK1/2 (pERK1/2), total ERK1/2 (ERK1/2), and β-actin (b) and quantification of activation of p38 (c) and ERK1/2 (d) are shown. n = 3 per group. * p < 0.05 and ** p < 0.01 versus control BMDMs (Veh). ## p < 0.01 versus stimulated BMDMs (LPS + LPA).