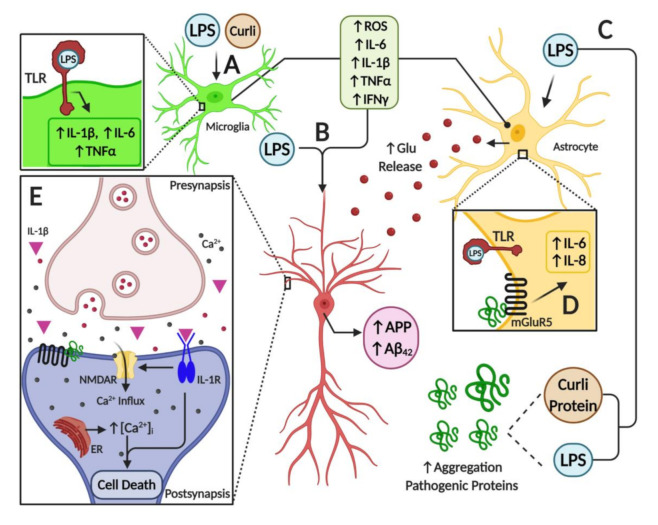
Figure 3.
Illustration of neuroinflammatory mechanisms mediated by microbiome-derived products in nervous tissue. (A) Toll-like receptors (TLRs) expressed in glial cells are activated by LPS, triggering the activation of astrocytes and microglial cells. This activation induces an inflammatory response by overexpression and release of pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α and IFN-γ, and by an increase in oxidative stress due to the generation of reactive oxygen species. Furthermore, bacterial amyloid proteins (curli) activate glial cells and induce the expression of pro-inflammatory mediators. (B) Pro-inflammatory mediators, together with LPS, increase the expression of the amyloid precursor protein (APP), and the deposition and misfolding of Aβ peptide. (C) Both LPS and curli are able to increase the deposition and aggregation of pathogenic proteins. (D) In astrocytes, among other cell types, activation of mGlurR5 receptor by pathogenic proteins triggers the overexpression of pro-inflammatory cytokines such as IL-6 and IL-8, which worsen the inflammatory milieu in the brain. Moreover, a high level of pro-inflammatory mediators leads to increased levels of the neurotransmitter glutamate, furthering ionic dyshomeostasis and augmenting neuronal excitotoxicity. (E) Finally, mGluR5 activation by pathogenic proteins induces the release of calcium from the endoplasmic reticulum, leading to ionic and mitochondrial dyshomeostasis, which results in neuron death. Furthermore, the activation of IL-1R in neurons by the binding of IL-1β cytokine amplifies the activity of NMDARs and mediates the inflammatory response via p38 MAPK. Overall, these alterations stimulate endoplasmic reticulum (ER) Ca2+ release through ryanodine receptors and IP3 receptors, which trigger ER stress and mitochondrial fragmentation leading to synaptic failure and neuronal apoptosis.