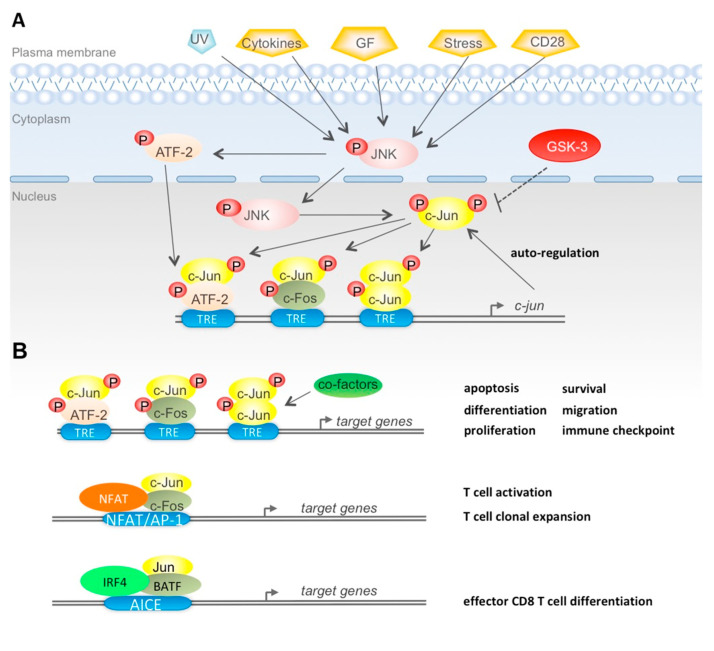
Figure 1.
(A) c-Jun/activator protein-1 (AP-1) regulation and biological activity. (A) c-Jun/AP-1 regulation via phosphorylation: application of various extracellular stimuli (UV irradiation, cytokines, growth factors, stress and CD8 signaling) activates JNK of the MAPK pathway through phosphorylation. Activated JNK (p-JNK) potentiates c-Jun via phosphorylation at sites in the N-terminal domain, which triggers either homodimerization of c-Jun or heterodimerization with c-Fos. P-JNK also phosphorylates/activates ATF-2 which forms dimers with c-Jun. The dimers bind to TRE elements along with co-factors (not shown) in order to activate transcription of the c-jun gene, thereby setting up an auto-regulatory mechanism of c-Jun/AP-1. On the other hand, GSK-3 phosphorylates c-Jun at sites in the C-terminal domain, thus, reducing respective gene transcription. (B) c-Jun/AP-1 biological outputs: in different types of mammal cells, c-Jun/AP-1 binds to TRE elements, along with co-factors (co-activators or co-repressors), in order to activate transcription of genes that regulate proliferation, differentiation, apoptosis, survival, migration of normal and malignant cells, as well as immune checkpoint function for cells of the immune system (upper scheme). In T cells, c-Jun/AP-1 forms ternary complexes with NFAT and binds NFAT/AP-1 composite sites present in the regulatory regions of cytokines and effector genes (middle scheme). In CD8 T cells, Jun/BATF form ternary complexes with IRF4 and AICE composite sites present in the regulatory regions of genes controlling effector differentiation of Ag-activated CD8 T cells (lower scheme).