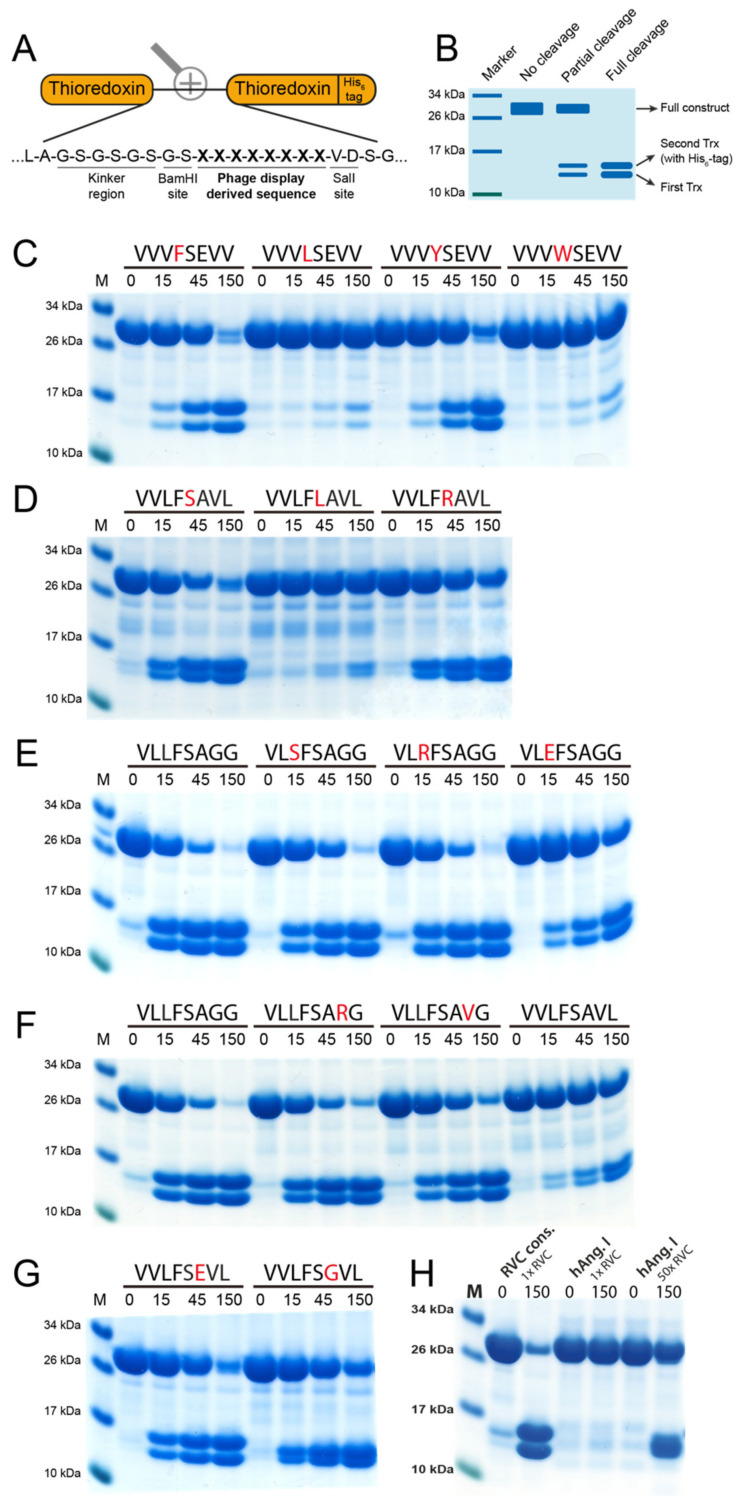
Figure 5.
Analysis of the consensus cleavage site of RVC using 2xTrx recombinant substrates. (A) Sequences derived from the phage display were inserted in the linking region between two E. coli thioredoxin (Trx) proteins, one of which has a C-terminal His6-tag. (B) Bands at ~28 kDa represent uncleaved two-Trx protein and the two bands present at ~14 kDa represent cleaved protein, with the upper of the two bands representing the Trx containing the His6-tag. Sequences above the panels represent the inserted octamers and numbers represent the reaction time in minutes. (C,D) display cleavage of substrates containing substitutions in positions P1 and P1’. Panels (E,F) show cleavage of the derived consensus sequence with substitutions in positions P2 and P3’, respectively. (G) Position P2’ was investigated using two different substrates. (H) The difference in amount of enzyme (RVC) needed to cleave Ang I in the 2xTrx context compared to the consensus site originating from the phage display (VLLFSAGG).