Abstract
This review, covering the literature from 1966 to the present (2020), describes naturally occurring fungal bioactive anthraquinones and analogues biosynthesized by the acetate route and concerning several different functionalized carbon skeletons. Hydrocarbons, lipids, sterols, esters, fatty acids, derivatives of amino acids, and aromatic compounds are metabolites belonging to other different classes of natural compounds and are generated by the same biosynthetic route. All of them are produced by plant, microorganisms, and marine organisms. The biological activities of anthraquinones and analogues comprise phytotoxic, antibacterial, antiviral, anticancer, antitumor, algicide, antifungal, enzyme inhibiting, immunostimulant, antiplatelet aggregation, cytotoxic, and antiplasmodium activities. The review also covers some practical industrial applications of anthraquinones.
Keywords: anthraquinones, natural analogues, fungi, biological activity
1. Introduction
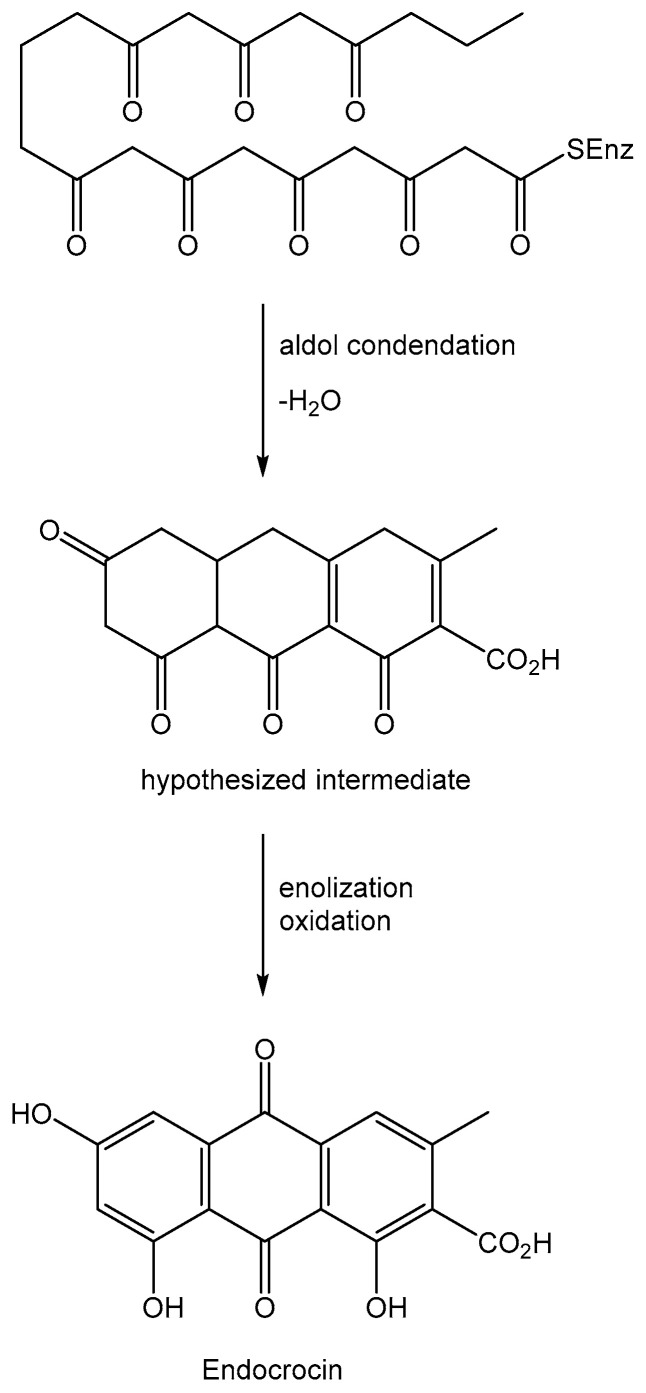
Anthraquinones are a group of natural compounds with a plethora of biological activities and potential practical applications. Most of them are produced by plant and micro-organisms among the living organisms [1,2]. They are acetate-derivative metabolites biosynthesized starting from a polyketide containing eight C2 units, which generates in turn with three aldol type condensations the carbon skeleton of anthraquinones except for the two carbonyl oxygens of the central ring. The latter are introduced by successive steps with an oxidation process. One example of this kind of biosynthesis is reported in Figure 1 for endocrin, a fungal anthraquinone produced by several Penicillium and Aspergillus species [3].
Figure 1.
Biosynthesis of the anthraquinone carbon skeleton.
Among secondary metabolites anthraquinones are the most investigated natural products for their mechanism of action [4]. Plants, microorganisms, lichens, and algae are producers of metabolites possessing diverse biological activities such as phytotoxic, antibacterial, antiviral, anticancer, antitumor, algicide, antifungal, enzyme inhibiting, immunostimulant, antiplatelet aggregation, cytotoxic and antiplasmodium activities. Anthraquinones are frequently reported among the plethora of different classes of natural compounds as alkaloids, hydrocarbons, lipids, sterols, esters, fatty acids, derivatives of amino acids, terpenoids, and aromatic compounds [5,6,7]. The activity of several hydroxyl- and amino-anthraquinones cannot be exploited due to their weak solubility in water. Thus, some of them are converted into water-soluble analogues by biotransformation [8]. Anthraquinones have also industrial application as natural dyes substituting synthetic chemicals in formulation to avoid undesired collateral effects [9].
Some previous reviews have described fungal anthraquinones such as that published by Gessler at al. (2013) [10], limited to 12 natural anthraquinones, and those on anthraquinones specifically produced by derived marine fungi [11]; also among them the treatment of the anthraquinones synthesized by a single fungal species such as Phoma was reported [12].
This review describes an advanced overview on anthraquinones, and related analogues grouped for the first time according to their natural sources. In particular, in addition to the isolation from fungal sources and their chemical characterization, their potential applications in different fields such as agriculture, medicine and the dyes industry are considered on the basis of their biological activities.
The first section chronologically describes the fungal anthraquinones starting from 1966 to the present day focusing on their sources, structures, and biological activities. The second section treats the industrial application of anthraquinones in a different field essentially as natural dyes. This part is focused on the comparison between natural and synthetic anthraquinone based dyes, their chemical derivatization and classification, and the advanced methods used in the treatment of the relative industrial wastewater to avoid severe negative environmental pollution. Finally, the main points described are summarized in the conclusion.
2. Fungal Anthraquinones and Analogues
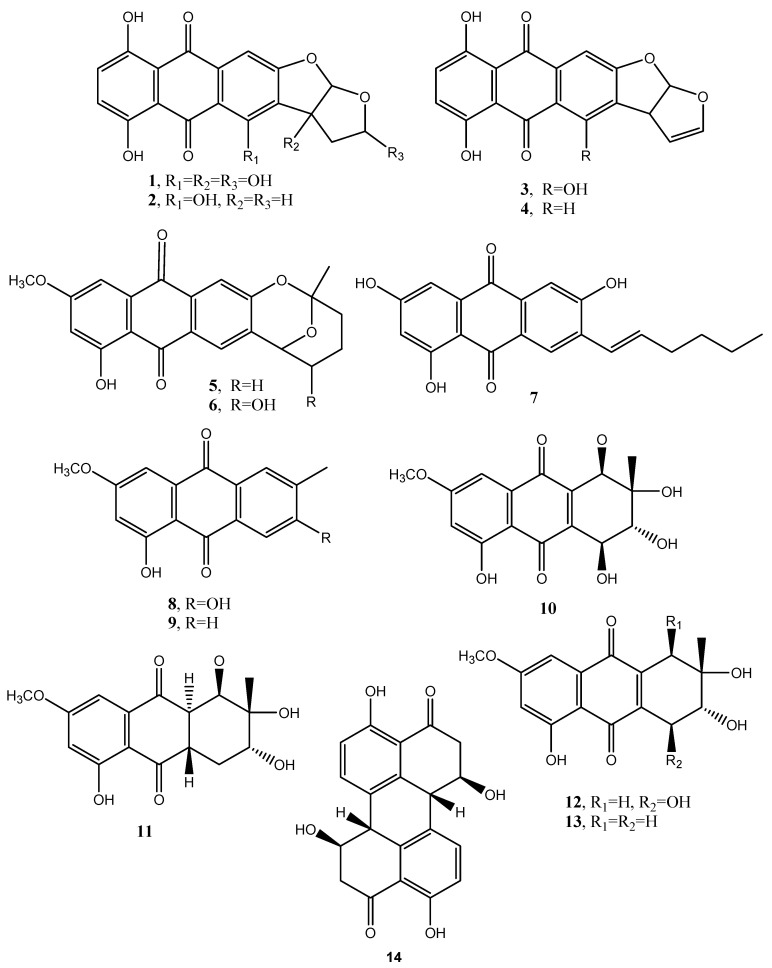
Dothistromin (1, Figure 2, Table 1) was isolated as the main phytotoxin produced by Dothistroma pini (Hulbary), a pathogen inducing necrotic disease characterized by the formation of red bands on the infected needles of Pinus radiata and other pines [13]. The same fungus also produced six other anthraquinones: bisdeoxydothistromin; bisdeoxydehydrodothistromin; 6-deoxyversicolorin C; averufin; nidurufin; averythrin (6–11, Figure 2, Table 1). No biological activity was reported for 2–7 [14]. More recently, averythrin was also isolated from the marine derived fungus Aspergillus versivolor [15].
Figure 2.
Bioactive anthraquinones and analogues produced by Dothistroma pini, Aspergillus versicolor, Alternaria porri, Alternaria solani, Alternaria cucumerina, Alternaria bataticola, Diaporthe angelicae and Stemphyfium botryosum.
Table 1.
Anthraquinones and analogues produced by phytopathogenic and endophytic fungi.
| Anthraquinone | Fungus | Biological Activity | Reference |
|---|---|---|---|
| Dothistromin (1, Figure 2) | Dothistroma pini | Phytotoxic | [13] |
| Bisdeoxydothistromin (2, Figure 2) | “ 1 | No activity | [14] |
| Bisdeoxydehydrodothistromin (3, Figure 2) | “ | “ | “ |
| 6-Deoxyversicolorin C (4, Figure 2) | “ | “ | “ |
| Averufin (5, Figure 2) | “ | “ | “ |
| Nidurufin (6, Figure 2) | “ | “ | “ |
| Averythrin (7, Figure 2) |
Dothistroma pini
Aspergillus versicolor |
“ | “ [15] |
| Macrosporin (8, Figure 2) |
Alternaria porri,
Alternaria solani, Alternaria cucumerina, Diaporthe angelicae, Stemphyfium botryosum |
Phytotoxic | [16,17,20] |
| 6-Methylxanthopurpurin 3 methyl eter (9, Figure 2) |
Alternaria bataticola
Alternaria solani |
“ | “ |
| Alternasolanol A (10, Figure 2) | D. angelicae | ” | [17] |
| Alternasolanol J (11, Figure 2) | “ | “ | “ |
| Stemphylin (12, Figure 2) | S. botryosum | Phytotoxic and weak antitumor | [20] |
| Dactylariol (13, Figure 2) | “ | Phytotoxic and in vitro anticancer | “ |
| Stemphyperylenol (14, Figure 2) | Stemphylium botryosum | Weak phytotoxic | [21] |
| Alterporriol (15, Figure 3) | “ | No activity | “ |
| Stemphylenol A (16, Figure 3) | “ | “ | “ |
| Stemphylenol B (17, Figure 3) | “ | “ | “ |
| Rugulosin (18, Figure 3) |
Cryphonectria parasitica
Hormonema dematioides |
Phytotoxic Insecticidal |
[22,24] |
| Skyrin (19, Figure 3) | ” | Phytotoxic | ” |
| Crysophanol (20, Figure 3) | “ | “ | “ |
| Emodin (21, Figure 3) |
C. parasitica, Pyrenophora tritici-repentis, Gliocladium sp. T31, Aspercgillus glaucus, H. dematioides, Aspergillus fumigatus, Phoma foevata |
Phytotoxic Mycotoxic Anticancer and inhibition in vitro DNA-dependent RNA polymerase |
[22,24,25,29,45,59,70] |
| 2-Chloroemodin (22, Figure 3) | Aspergillus fumigatus | No activity | [25] |
| Physcion (23, Figure 3) | “ Microsporum sp. |
Anticancer | “ [26] |
| Catenarin (24, Figure 3) |
Drechslera teres, Drechslera graminea, Drechslera tritici-repentis, Drechslera phlei, Drechslera dictyoides, Drechslera avenae
Aspergillus cristatus |
Antibiotic against Gram+ Phytotoxic Anticancer and inhibition in vitro DNA-dependent RNA polymerase |
[28,29,70] |
| Helminthosporin (25, Figure 3) |
D. avenae,
Bipolaris sorokiniana |
No activity | [28.29] |
| Cynodontin (26, Figure 3) | “ | No activity | “ |
| Cytoskyrin A (27, Figure 3) | Cytospora sp. CR200 | BIA | [30,31] |
| Cytoskyrin B (28, Figure 3) | “ | BIA | “ |
| Luteoskyrin (29, Figure 3) | “ | No activity | “ |
| Dendryol A (30, Figure 4) | Dendryphiella sp. | Phytotoxic | [35] |
| Dendryol B (31, Figure 4) | “ | “ | “ |
| Dendryol C (32, Figure 4) | “ | “ | “ |
| Dendryol D (33, Figure 4) | “ | “ | “ |
| Rubellin A (34, Figure 4) | Ramularia collo-cygni | Increased photodynamic oxygen activation | [37,38] |
| Rubellin B (35, Figure 4) | Phytotoxic, antibiotic, antiproliferative, and cytotoxic | [38,39] | |
| Rubellin C (36, Figure 4) | “ | “ | “ |
| Rubellin D (37, Figure 4) | “ | “ | “ |
| Rubellin E (38, Figure 4) | “ | “ | “ |
| Rubellin F (39, Figure 4) | “ | No activity | “ |
| Uridinetubellins I (40, Figure 4) |
Ramularia uredinicola
Ramularia collo-cygni |
The photodynamic action toward three mammalian cell lines | [40] |
| Uridinetubellins II, (41, Figure 4) | “ | “ | “ |
| Caeruleoramularin (42, Figure 4) | “ | No activity | “ |
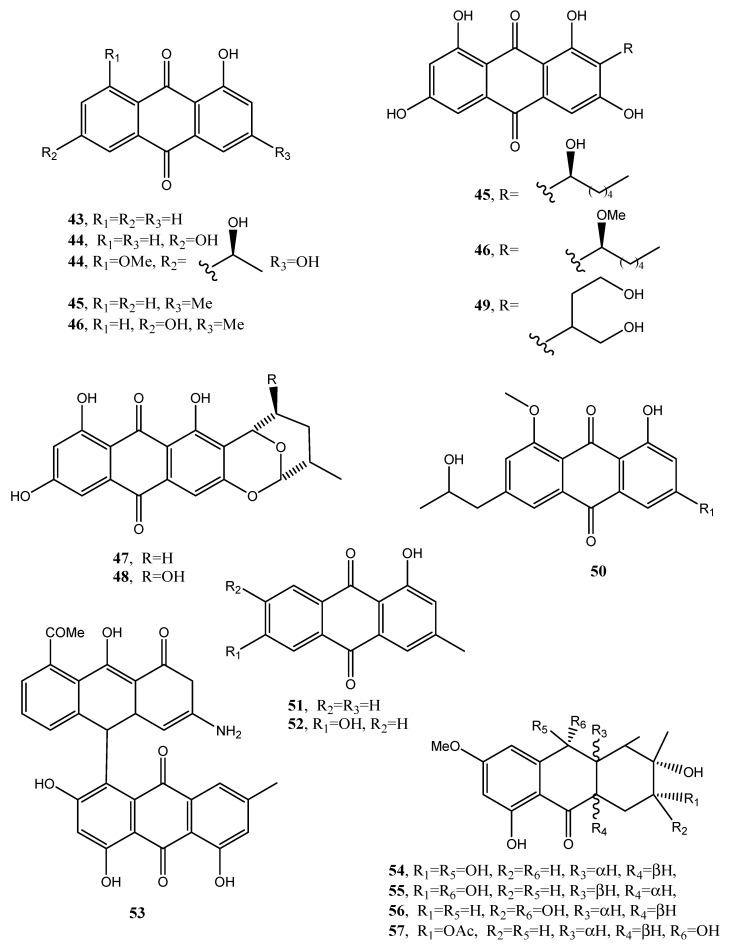
| 1-Hydroxy-3-methyl-anthraquinone (43, Figure 4) | Trichoderma harzianum | No activity | [41] |
| 1,8-Dihydroxy-3-methyl-anthraquinone (44, Figure 4) | “ | “ | “ |
| Averantin (45, Figure 5) | Aspergillus versicolor | Antibiotic, Cytotoxic |
[42,45,48] |
| Methyl-averantin (46, Figure 5) | “ | Cytotoxic | “ |
| Averufin (47, Figure 5) | “ | No activity | “ |
| Nidurufin (48, Figure 5) | “ | Antibiotic and cytotoxic | “ |
| Versiconol (49, Figure 5) | “ | No activity | “ |
| Isorhodoptilometrin-1-methyl ether (50, Figure 5) | “ | Antibiotic and mild anticancer | [43] |
| Pachybasin (51, Figure 5) |
P. foevata, Coniothyrium sp., Ascochyta lentis |
Weak antibiotic Antibiotic |
[45,71,78] |
| Phomarin (52, Figure 5) | “ | Antifungal activity Antibiotic |
[45,71,78] |
| Anhydropseudophlegmacin-9,10-quinone-3′-amino-8′-O-methyl ether (53, Figure 5) | Phoma herbarum | Phytotoxic | [46] |
| Tetrahydroaltersolanol C (54, Figure 5) | Alternaria sp. | Antiviral | [47] |
| Tetrahydroaltersolanol D (55, Figure 5) | “ | No activity | “ |
| Tetrahydroaltersolanol E (56, Figure 5) | “ | “ | “ |
| Tetrahydroaltersolanol F (57, Figure 5) | “ | “ | “ |
| Dihydroaltersolanol A (58, Figure 6) | “ | “ | “ |
| Alterporriol N (59, Figure 6) | “ | “ | “ |
| Alterporriol O (60, Figure 6) | “ | “ | “ |
| Alterporriol P (61, Figure 5) | “ | Cytotoxic | “ |
| Alterporriol Q (62, Figure 6) | “ | Antiviral | “ |
| Alterporriol R (63, Figure 6) | “ | No activity | “ |
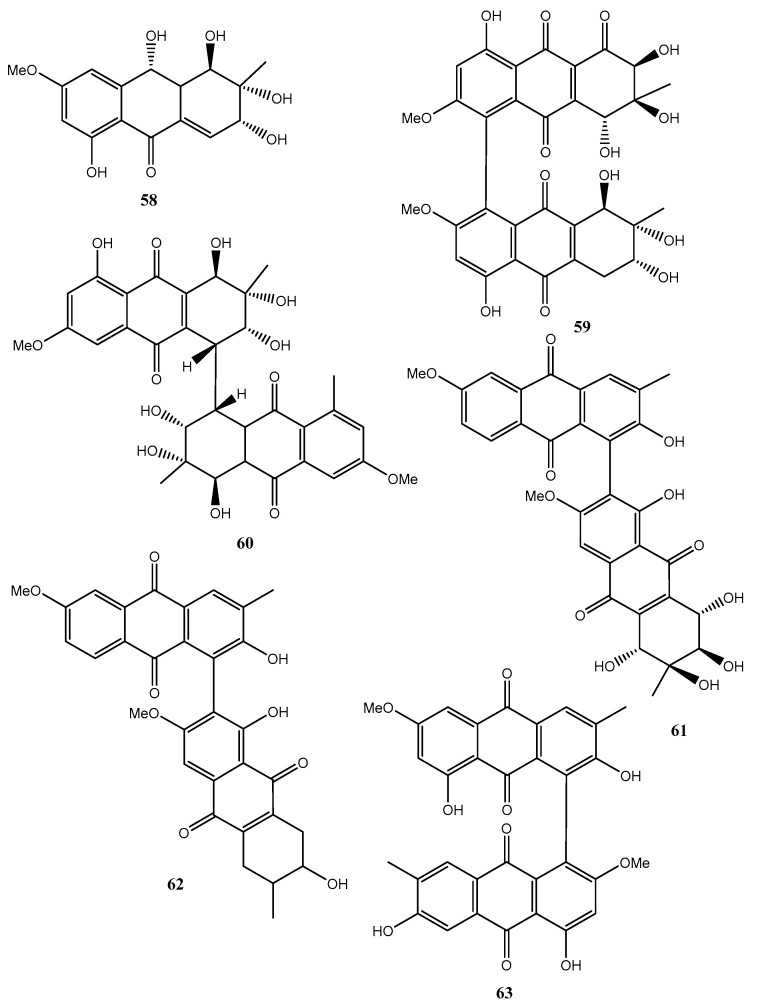
| Holoroquinone (64, Figure 7) | Halorosellinia sp. | Antitumor | [48] |
| Torrubiellin A (65, Figure 7) | Torrubiella sp. BCC 28517 | Moderate antimalarial, antifungal, antibacterial, cytotoxic | [49] |
| Torrubiellin A (66, Figure 7) | “ | Antimalarial, antifungal, antibacterial, cytotoxic | “ |
| Acremoxanthone C (67, Figure 7) | Hypocreales sp. MSX 17022 | Moderate cytotoxic | [50] |
| Acremoxanthone D (68, Figure 7) | “ | Moderate cytotoxic, and moderate 20S proteosome inhibition | “ |
| Penicillanthranin A (69, Figure 7) | Penicillium citrinum PSU-F51 | Moderate antibacterial and mild cytotoxic | [51] |
| Penicillanthranin B (70, Figure 7) | “ | No activity | “ |
| Auxarthrol C (71, Figure 7) | Stemphylium sp. 33231 | “ | [52] |
| Macrosporin 2-O-(6′-acetyl)-α-d-glucopyranoside (72, Figure 7) | “ | “ | “ |
| 2-O-Acetylaltersolanol B (73, Figure 8) | “ | “ | “ |
| 2-O-Acetylaltersolanol L (74, Figure 8) | “ | “ | |
| Alterporriols T (75 Figure 8) | “ | “ | “ |
| Alterporriols U (76 Figure 8) | “ | “ | “ |
| Alterporriols V (77 Figure 8) | “ | “ | “ |
| Alterporriols W (78 Figure 8) | “ | Weak antibacterial and moderate zootoxic | “ |
| 6,8,1′-Tri-O-methyl averantin (79, Figure 8) |
Penicillium purpurogenum Endophytic fungus ZSUH-36 |
Zootoxic and antifungal |
[53,54] |
| 6,8-Di-O-methyl averufnin (80, Figure 8) |
Penicillium purpurogenum Endophytic fungus ZSUH-36 Aspergillus versicolor |
No activity |
[53,54,56] |
| 6,8-Di-O-methyl averufanin (81, Figure 8) | “ |
Antibiotic and zootoxic |
[53,54,56] |
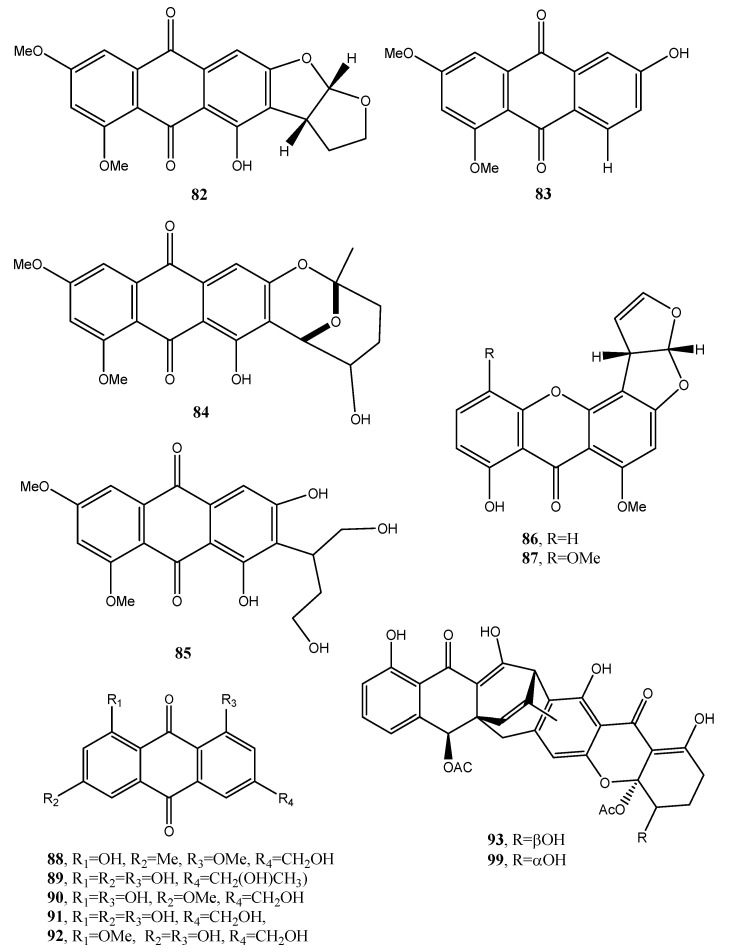
| Aversin (82, Figure 9) | “ |
Antifungal |
[53,55,56] |
| 1,3-Dihydroxy-6,8-dimethoxy-9,10-anthraquinone (83, Figure 9) | Penicillium purpurogenum | No activity | [53] |
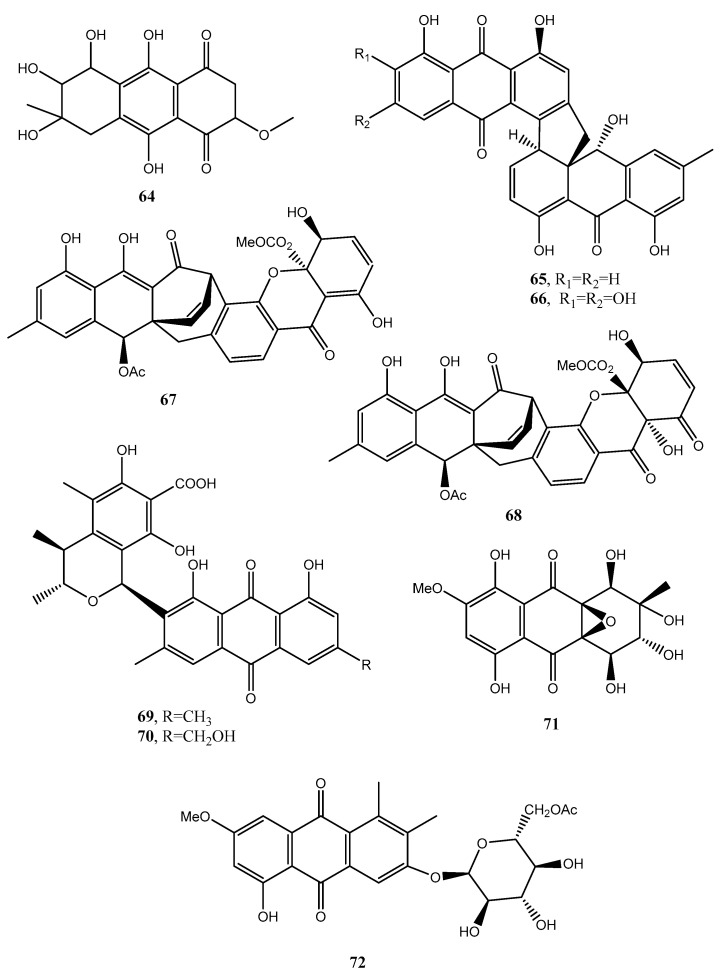
| 6,8-Di-O-methylnidurufin (84, Figure 9) |
Penicillium purpurogenum Endophytic fungus ZSUH-36 Aspergillus versicolor |
Antifungal and phytotoxic |
[53,54,56] |
| 6,8-Di-O-methyl versiconol (85, Figure 9) |
Penicillium purpurogenum Endophytic fungus ZSUH-36 |
Antifungal and phytotoxic |
[53,55] |
| 5-Methyoxysterigmatocystin (86, Figure 9) | “ | Zootoxic | [53] |
| Sterigmatocystin (87, Figure 9) | Penicillium purpurogenum | No activity | [55] |
| Questin (88, Figure 9) |
Aspergillus sp. YL-6, Polygonum cuspidatum |
Allelopathy |
[57,58] |
| Isorhodoptilometrin (89, Figure 9) |
Aspergillus sp. YL-6, Gliocladium sp. T31 |
Alleopathy |
[57,59] |
| Fallacinol (90, Figure 9) | Polygonum cuspidatum | No activity | [58] |
| Citreorosein (91, Figure 9) |
Polygonum cuspidatum Gliocladium sp. T31 |
“ | [58,59] |
| Questinol (92, Figure 9) | Gliocladium sp. T31 | “ | [58] |
| Engyodontochone A (93, Figure 9) | Engyodontium album | Antibiotic | [60] |
| Engyodontochone B (94, Figure 10) | “ | No activity | “ |
| Engyodontochone C (95, Figure 10) | “ | Antibiotic | “ |
| Engyodontochone D (96, Figure 10) | “ | “ | “ |
| Engyodontochone E (97, Figure 10) | “ | “ | “ |
| Engyodontochone F (98, Figure 10) | “ | “ | “ |
| Betacolin-like compound (99, Figure 9) | “ | Antibiotic | “ |
| JBIR-99 (100, Figure 10) | “ | No activity | “ |
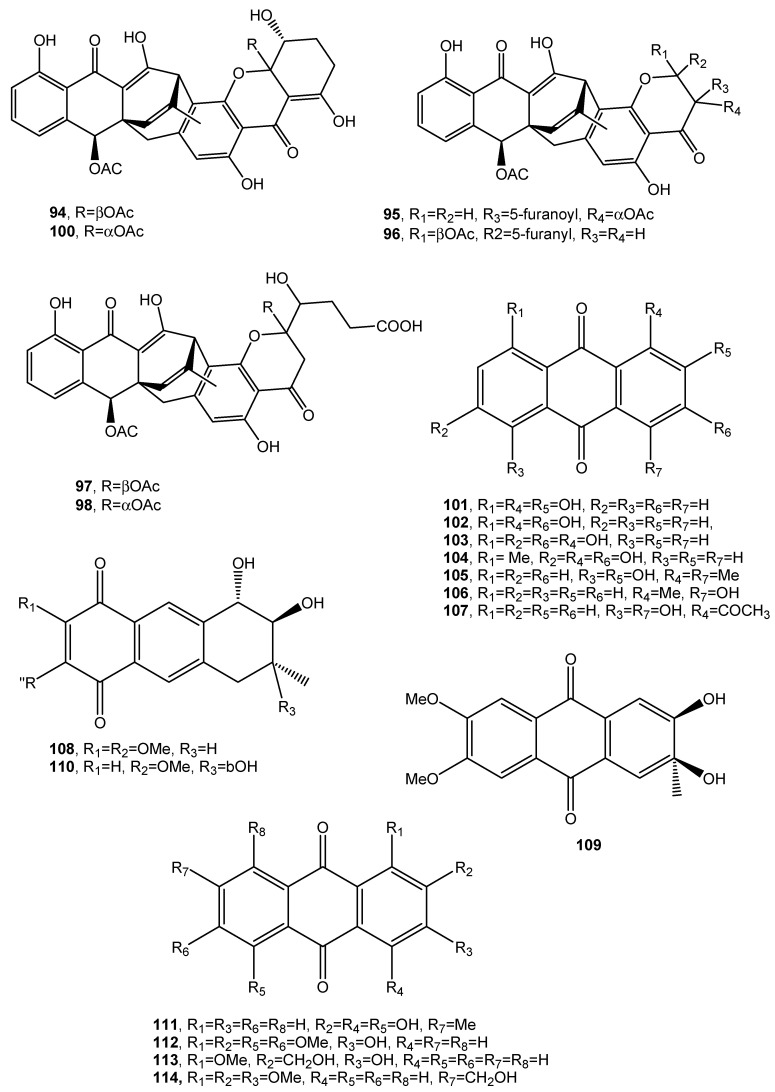
| 1,2,8-Trihydroxyanthraquinone (101, Figure 10) | Nigrospora sp. | “ | [61] |
| 1,3,8-Trihydroxyanthraquinone (102, Figure 10) | “ | Antifungal | “ |
| 1,3,6-trihydroxy-8-methylanthraquinone (103, Figure 10) | “ | “ | “ |
| Rheoemodin (104, Figure 10 | “ | Antimicrobial | “ |
| Aloesaponarin II (105, Figure 10) | “ | Antifungal | “ |
| Isozyganein (106, Figure 10) | “ | Antioxidant | “ |
| 1-Acetyl-4,5-dihydroxy-anthraquinone (107, Figure 10) | “ | No activity | |
| Aspetritone A (108, Figure 10) | Aspergillus tritici | Strong antibiotic and cytotoxic activity | [63] |
| Aspetritone B (109, Figure 10) | “ | No activity | “ |
| Bostrocyn (110, Figure 10) | “ | “ | “ |
| Compound 111 (Figure 10) | “ | “ | “ |
| Compound 112 (Figure 10) | “ | “ | “ |
| Compound 113 (Figure 10) | “ | “ | “ |
| Compound 114 (Figure 11) | “ | “ | “ |
| 1-O-methyl-6-O-(α-d-ribofuranosyl)-emodin (115, Figure 11) | Gaeumannomyces sp. | Anti-inflammatory Reduction of NO production by LPS- | [64] |
| 1-O-Methylemodin (116, Figure 11) |
Gaeumannomyces sp. Phialophora alba |
Anti-inflammatory Reduction of NO production by LPS Growth inhibition of Phellinus tremulae Inhibition of the secretion of IL-625 Protein tyrosine phosphatase 1B inhibition |
[64,65,66,67,68] |
| 5-Chloro-6,8-dihydroxy-1-methoxy-3-methylanthraquinone (117, Figure 11) | Phialophora alba | No activity | [65] |
| 7-Chloro-6,8-dihydroxy-1-methoxy-3-methylanthraquinone (118, Figure 11) | “ | “ | “ |
| 5-Chloro-6,8,10-trihydroxy-1-methoxy-3-methyl-9(10H) anthracenone (119, Figure 11) | “ | “ | “ |
| 5-chloro-8,10-dihydroxy-l,6-dimethoxy-3-metbyl-9(10H)-anthracenone (120, Figure 11) | “ | “ | “ |
| Rubrumol (121, Figure 11) | Eurotium rubrum | Activity when tested on Topo I | [69] |
| Rubrocristin (122, Figure 11) | “ Aspergillus glaucus |
No Activity | “ |
| 2-Methyleurotinone (123, Figure 11) | Eurotium rubrum | “ | “ |
| Conyothyrinone A (124, Figure 11) |
Eurotium rubrum Coniothyrium sp. |
Antifungal activity | [69,70,71] |
| Erythroglaucin (125, Figure 11) | Aspergillus glaucus | No activity | [70] |
| Physcion-9-anthrone (126, Figure 11) | “ | “ | “ |
| Viocristin (127 Figure 11) | “ | Antibacterial activity Anticancer activity |
“ |
| Isoviocristin (128, Figure 11) | “ | Antibacterial activity | “ |
| Conyothyrinone B (129, Figure 11) | Coniothyrium sp. | Antimicrobial activity | [71] |
| Conyothyrinone C (130, Figure 11) | “ | “ | “ |
| Conyothyrinone D (131, Figure 11) | “ | “ | “ |
| 1,7-Dihydroxy-3-methyl-9,10-anthraquinone (132, Figure 11) | “ | Antimicrobial activity and strong antibacterial activity | “ |
| 1-Hydroxy-3-hydroxymethyl-9,10-anthraquinone (133, Figure 11) | “ | Antimicrobial | “ |
| (–)-2′R-1-hydroxyisorhodoptilometrin (134, Figure 11) |
Penicillium sp. OUCMDZ-4736 |
Anti-hepatitis B virus | [72,73] |
| Methyl 3,4,8-trihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate. (135, Figure 11) | “ | No activity | [72] |
| Methyl 6,8-dihydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylate (136, Figure 11) |
“ | “ | “ |
| Danthron (137, Figure 11) | Paraconiothyrium sp. | Antibacterial, antifungal and anticancer | [74] |
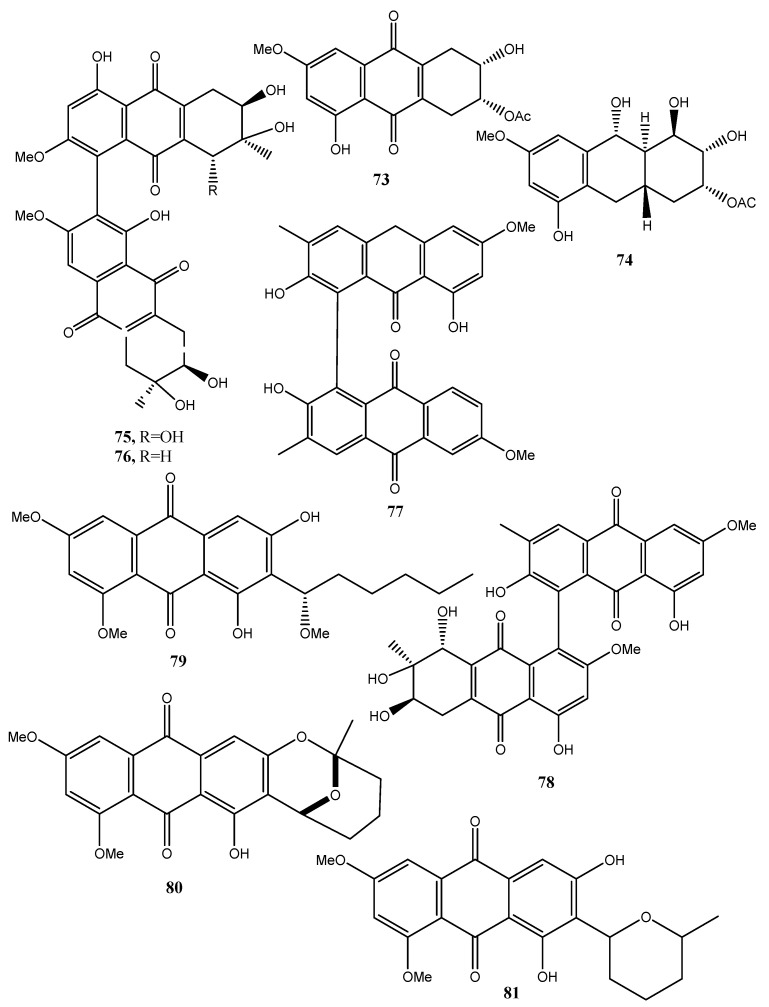
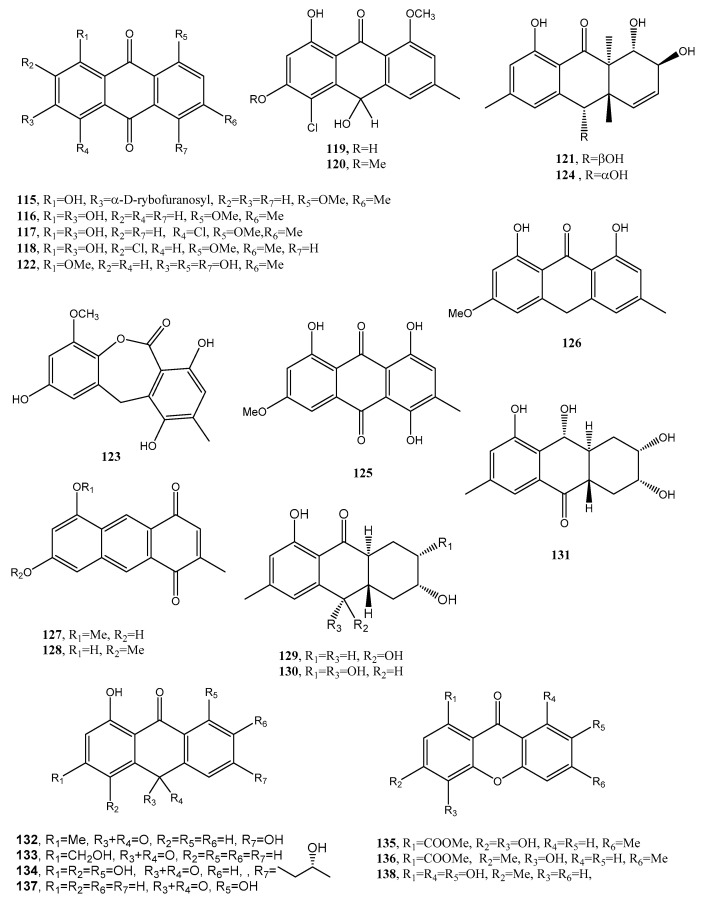
| Bostrycoidin (138, Figure 12) | Fusarium solani | Antimicrobial and anticancer | [77] |
| Lentiquinones A (139, Figure 12) | Ascochyta lentis | Phytotoxic and antimicrobial | [78] |
| Lentiquinones B (140, Figure 12) | “ | “ | “ |
| Lentiquinones C (141, Figure 12) | “ | “ | “ |
| Lentisone (142, Figure 12) | “ | “ | “ |
| ω-Hydroxypachybasin (143, Figure 12 | “ | “ | “ |
| 1,7-Dihydroxy-3-methylanthracene-9,10-dione (144, Figure 12) | “ | “ | “ |
| Anthraquinone dimer (145, Figure 12) | Aspergillus versicolor | Selective antibacterial | [15] |
| Anthraquinone dimer (146, Figure 12) | “ | “ | “ |
| 1′-O-Methylaverantin (147, Figure 12) | “ | No activity | “ |
| Averantin (148, Figure 12) | “ Aspergillus sp. SCSIO F063 |
“ |
“ [80] |
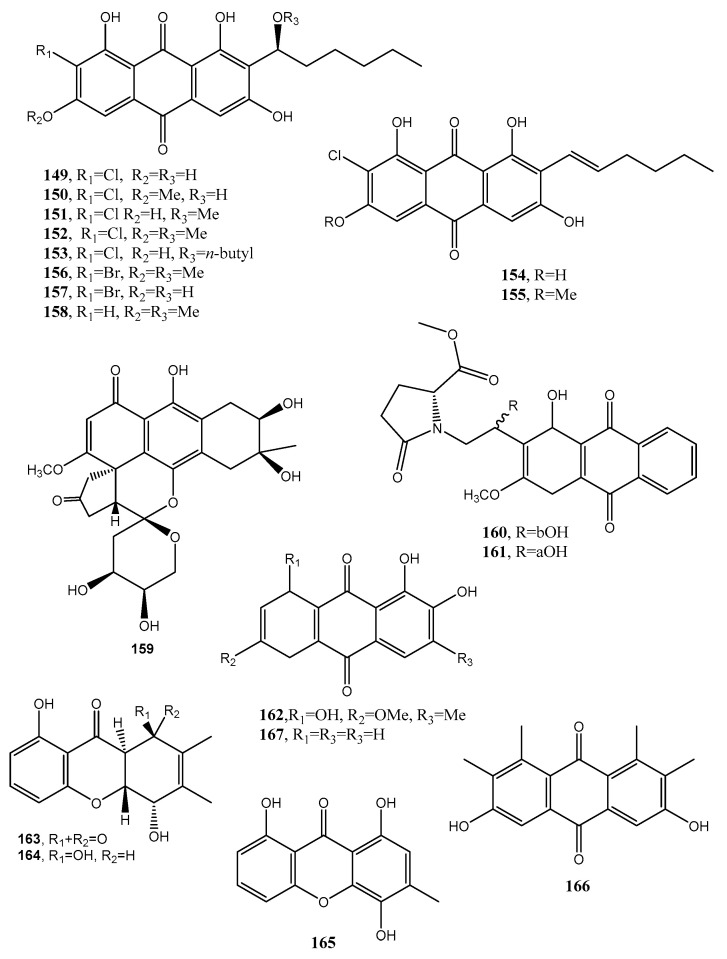
| (1′S)-7-Chloroaverantin (149, Figure 13) | Aspergillus sp. SCSIO F063 | “ | [80] |
| (1′S)-6-O-Methyl-7-chloroaverantin (150 Figure 13) | “ | “ | |
| (1′S)-1′-O-Methyl-7-chloroaverantin (151, Figure 13) | “ | “ | “ |
| (1′S)-6,1′-O,O-Dimethyl-7-chloroaverantin (152, Figure 13) | “ | “ | “ |
| (1′S)-7-Chloroaverantin-1′-butyl ether (153, Figure 13) | “ | “ | “ |
| 7-Chloroaverythrin (154, Figure 13) | “ | “ | “ |
| 6-O-Methyl-7-chloroaverythrin (155, Figure 13) | “ | Anticancer | “ |
| (1′S)-6,1′-O,O-Dimethyl-7-bromoaverantin (156, Figure 13) | “ | No activity | “ |
| and (1′S)-6-O-Methyl-7-bromoaverantinone (157, Figure 13) | “ | “ | “ |
| (1′S)-6,1′-O,O-Dimethylaverantin (158, Figure 13) | “ | “ | “ |
| Anthrininone A (159, Figure 13) | Alternaria tenuissima | Inhibition activity against indoleamine 2,3-dioxygenase and stimulate intracellular calcium levels | [81] |
| Anthrininone B (160, Figure 13) | “ | Inhibition activity against indoleamine 2,3-dioxygenase and against different protein tyrosine phosphatases | “ |
| Anthrininone C (161, Figure 13) | “ | “ | “ |
| 6-O-Methylalaternin (162, Figure 12 | “ | “ | “ |
| Funiculosone (163, Figure 13) | Talaromyces funiculosus | Antimicrobial | [82] |
| Mangrovamide J (164 Figure 13) | “ | “ | “ |
| Ravenelin (165 Figure 13) | “ | “ | “ |
| Neoanthraquinone (166, Figure 13) | Neofusicoccum luteum | Phytotoxic | [83] |
| Alizarin (167, Figure 13) | Rubia tinctorum | Dye | [84] |
1 This menas that the table cells contain the same concept.
Macrosporin and 6-methylxanthopurpurin 3-methyl ether (8 and 9, Figure 2, Table 1) are two anthraquinones produced by Alternaria bataticola, the causal agent of a black spot of sweet potato [16]. Compound 8 was also isolated from other fungi of the same genus as A. porri, A. solani and A. cucumerina while 9 was isolated also from A. solani [16]. Then macrosporin was isolated together with another two anthraquinones, named altersolanols A and J (10 and 11, Figure 2, Table 1), as well as nectriapyrone, an α-pyrone, from the culture filtrates of Diaporthe angelicae (anamorph Phomopsis foeniculi), the causal agent of serious disease on fennel (Foeniculum vulgare) in Bulgaria. These four metabolites were tested on detached tomato leaves and only nectriapyrone and altersolanols A and J showed a modulate phytotoxicity while macrosporin was not toxic [17].
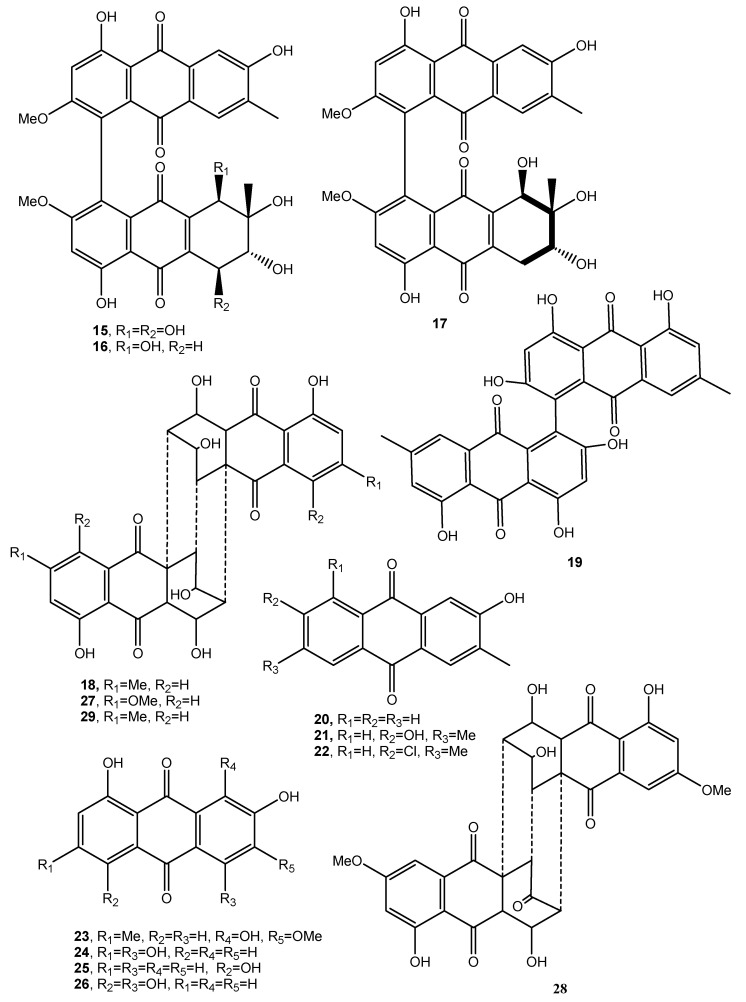
Stemphylin, is a phytotoxin (12, Figure 2, Table 1) produced by Stemphyfium botryosum, a fungal pathogen inducing a destructive disease in lettuce. The first structure of compound 12 was wrongly assigned by Barash et al. (1975 and 1978) [18,19], and then corrected when it was isolated from the same fungus together with two other phytotoxic anthraquinones, the above cited macrosporin and dactylariol (13, Figure 2, Table 1) [20]. The latter compound (13) showed anti Adenosine TriPhosphate (ATP) catabolism in Erlich I ascite tumor cells while stemphylin showed a weak antitumor activity on the treated animal at a dose of 40 mg/kg [20]. Stemphylium botryosum, inducing leaf spot disease on beet plants, also synthesized macrosporin and dactylariol (8 and 13), together with other anthraquinones identified as stemphyrperylenol (14, Figure 2), alteroporiol, and stemphynols A and B (15–17, Figure 3, Table 1). The phytotoxicity of all the metabolites (8, 13–17) was tested on lettuce and beet evaluating the seeds elongation. Compound 13 was the most active while compound 14 exhibited a moderate inhibition while the other ones were inactive [21].
Figure 3.
Bioactive anthraquinones and analogues produced by Stemphyfium botryosum, Cryphonectria parasitica, Hormonema dematioides, Microsporum sp., Aspergillus claucus, Pyrenophora tritici-repentis, Gliocladium sp. T31, Aspergillus fumigatus, Drechslera teres, Drechslera graminea, Drechslera tritici-repentis, Drechslera phlei, Drechslera dictyoides, Drechslera avenae, Bipolaris sorokinana and Cytospora CR200.
Cryphonectria (Endothia) parasitica (Murr.) Barr, the causal agent of chestnut (Castanea sativa) canker disease and other species produces diaporthin, a phytotoxic benzopyranone pigment, together with phytotoxic anthraquinones. The hundreds of fungal strains were grouped into virulent, intermediate and hypervirulent and produced, respectively, diaporthin, rugulosin and skirin (18 and 19, Figure 3, Table 1), crysophanol (20, Figure 3, Table 1) and emodin (21, Figure 3, Table 1) [22]. Virulent and hypovirulent strains of C. parasitica also produced a main polysaccharide identified as pullulan (a polymer constituted of α-1,4- and α-1,6-glucan) and a minor fraction which gave phytotoxicity on both the host plant and tomato (Lycopersicon escultem L.) leaves. One component of this minor fraction was identified as a galactan and consisted of the repeat unit: [→6)-β-d-Galf-(1→5)-β-d-Galf-(1→]n [23].
Rugulosin (18) was isolated together with emodin and skyrin from Hormonema dematioides and showed activity against survival of budworm larvae while the other two anthraquinones were inactive [24].
Aspergillus fumigatus, which is responsible for a lung disease, produced a plethora of secondary metabolites belonging to different classes of natural compounds. Among them, emodin, 2-chloro-emodin (21 and 22 Figure 3), and physcion (23, Figure 3) were also isolated. However, compound 23 appeared not to have a role in the fungal infection [25].
Physcion (23) was also isolated from the organic culture extract of the marine derived fungus Microsporum sp. and showed a cytotoxic effect on human cervical carcinoma HeLa cells and its apoptosis induction was deeply investigated. Physcion also caused the formation of reactive oxygen species (ROS) in the same cells [26].
Many Drechslera species, which are important pathogens on gramineous plants and their seeds, produced colored pigments [27]. The red pigment exudated from Drechslera teres, D. graminea, D. tritici-repentis, D. phlei, D. dictyoides, D. avenae was identified as the anthraquinone catenarin (24, Figure 3, Table 1) while that from D. avenae and Bipolaris sorokiniana were two other anthraquinones recognized as helminthosporin and cynodontin (25 and 26, Figure 3, Table 1). Catenarin (24) showed a total inhibition of Bacillus subtilis (Gram+) growth but had no effect on the Gram- bacterium Ervinia carotova but in part inhibited the mycelium growth of D. teres [28]. In particular, catanerin and emodin (24 and 21) were also found in kernels infected by Pyrenophora tritici-repentis (Died.) Drechs. (anamorph: Drechslera tritici-repentis (Died.) Shoem.), the causal agent of tan spot of wheat. Compound 24 caused the reddish discoloration with red smudge of kernels, while compound 21 indicated that P. tritici-repentis is a mycotoxigenic fungus. Compound 24 also induced non-specific leaf necrosis and appeared moderately active against some of the fungi associated with P. tritici-repentis suggesting its possible role in the life strategy of the pathogen [29].
Cytoskyrins A and B (27 and 28, Figure 3, Table 1) are two closely related bisanthraquinones obtained from large-scale cultures of an endophytic fungus, CR200 (Cytospora sp.), isolated from the branch of a Conocarpus erecta tree in the Guanacaste Conservation Area of Costa Rica. Previously, a substituted benzopryrone, named cytosporone, was isolated from the same fungus and showed antibiotic activity [30]. Cytoskyrin A showed strong BIA activity down to 12.5 ng in the standard assay while cytoskyrin B was inactive at the concentrations tested (<50 mg) [31]. The biochemical induction assay (BIA) measures the induction of the SOS response in bacteria and is used to identify compounds that inhibit DNA synthesis, either directly by inhibiting the DNA replication machinery or more often indirectly by modifying DNA [32,33,34]. BIA activity is highly dependent on the three-dimensional structure and not a general property of these polyphenolic compounds. In fact, close bisanthraquinones such as luteoskyrin (29, Figure 3) and rugulosin (18) are known to interact with DNA. The structural basis of these differences is not yet clear [31].
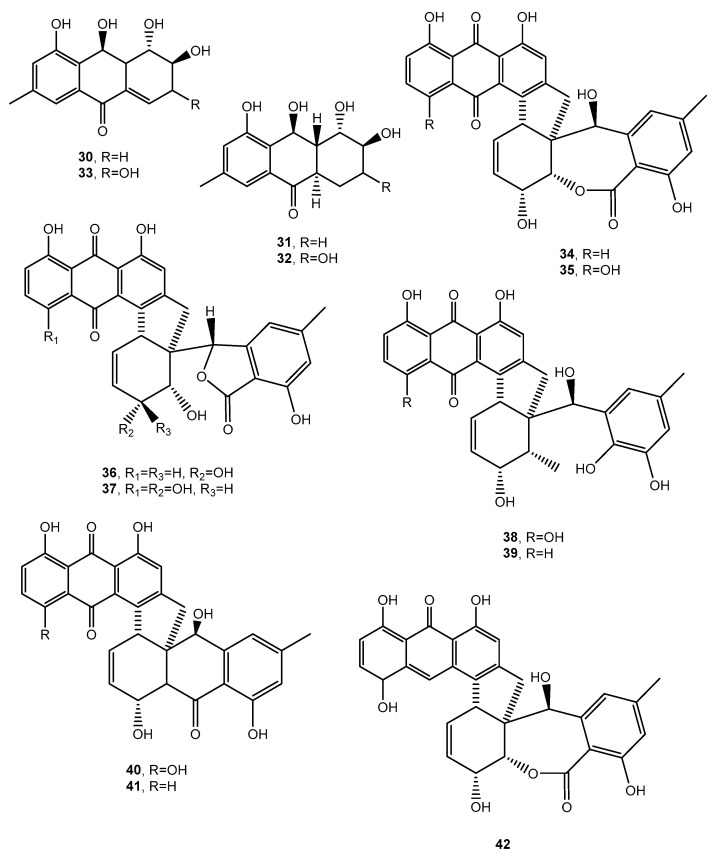
Dendryols A–D (30–33, Figure 4, Table 1), four phytotoxic anthraquinones, were produced by Dendryphiella sp. [35], a fungus isolated from an infected sample of the paddy weed Eleocharis kuroguwai (Cyperaceae) in Japan [36]. The dendryols 30–33, when tested for the phytotoxic activity by leaf-puncture assay on weeds (kuroguwai, barnyardgrass, and velvetleaf) and cultivated crops (rice, corn, and cowpea), showed toxicity only against barnyardgrass and the nercrotic area appeared to be dose-dependent. Compound 30 caused similar necrosis only on velvetleaf [35].
Figure 4.
Bioactive anthraquinones and analogues produced by Dendryphiella sp., Ramularia collo-cygni, Trichoderma harzianum and Ramularia uredinicola.
Rubellin A (34, Figure 4, Table 1) was isolated from the culture filtrates of Ramularia collocygni, the causal agent of leaf-spot disease of barley in central Europe [37]. From the same fungus rubellins B–F and 14-deydro rubellin D (35–38, Figure 4, Table 1) were also isolated. Biosynthetic studies carried out by the incorporation of both [1-13C]-acetate and [2-13C]-acetate into the rubellins demonstrated that such anthraquinone derivatives were biosynthesized via the polyketide pathway. Rubellin A (34) increased photodynamic oxygen activation [38], while rubellins B–E exhibited antibacterial activity, as well as light-dependent, antiproliferative and cytotoxic activity in a series of human tumor cell lines [39]. Closely related anthraquinones were isolated from the same fungus and from Ramularia uredinicola and identified as uridinetubellins I and II, and caeruleoramulin (40–42, Figure 4 and Table I). Both uredinorubellins (40 and 41) showed photodynamic activity comparable to rubellin D, whereas caeruleoramularin did not display such activity [40].
1-Hydroxy-3-methyl-anthraquinone and 1,8-dihydroxy-3-methyl-anthraquinone (43 and 44, Figure 5, Table 1), were isolated together with other bioactive metabolites from Trichoderma harzianum in a study aimed to improve the production and application of novel biopesticides and biofertilizers and thus to help in the management of crop plant diseases. However, the two anthraquinones had no role in this activity [41].
Figure 5.
Bioactive anthraquinones and analogues produced by Aspergillus versicolor, Phoma foevata, Coniothyrium sp., Phoma herbarum, Ascochyta lentis and Alternaria sp.
Five anthraquinones named averantin, methyl-averantin, averufin, nidurufin, and versiconol (45–49, Figure 5, Table 1) were isolated from Aspergillus versicolor, a sponge-derived fungus. Methyl averatin and nidurufin (45 and 48) showed strong cytotoxicity against five human solid tumor cell lines (A-549, SK-OV-3, SK-MEL-2, XF-498, and HCT-15) with IC50 values in the range of 0.41–4.61 μg/mL.
Averatin and nidurufin (45 and 48) had antibacterial activity against several clinical isolates of Gram + strains with MIC values of 0.78–6.25 μg/mL [42]. Successively, a new anthraquinone, named isorhodoptilometrin-1-methyl ether (50, Figure 5, Table 1), was isolated together with the already known emodin, 1-methyl emodin, evariquinone, 7-hydroxyemodin, 6,8-methyl ether, siderin, arugosin C, and variculanol from the same fungus but obtained from the Red Sea green alga Halimeda opuntia. Compound 50 and siderin showed moderate antibacterial activity against B. subtilis, B. cereus, and S. aureus at 50 μg/disk, while, tested at 3 μg, only compound 50 exhibited mild solid tumor selectivity HEP-G2 with respect to the human normal cells (CFU) [43].
Phoma is a genus well known as producer of phytotoxin belonging to different classes of natural compounds including anthraquinones [44]. In fact, from Phoma foevata, the causal agent of gangrene in potatoes, several anthraquinones were isolated such as the already cited emodin and crysophanol, together with pachybasin and phomarin (51 and 52, Figure 5, Table 1) but no biological activity was reported [45]. Furthermore, a phytotoxic anthraquinone, which was identified as anhydropseudophlegmacin-9,10-quinone-3′-amino-8′-O-methyl ether (53, Figure 5, Table 1), was isolated from Phoma herbarum FGCC#54, a phytopathogenic fungus, investigated for herbicidal potential. Compound 53 showed phytotoxic activity against the prominent weeds of Central India [46].
Fungi belonging to the Alternaria genus are also well known as producers of a plethora of bioactive metabolites. In fact, five new hydroanthraquinone derivatives, named tetrahydroaltersolanols C–F (54–57, Figure 6, Table 1) and dihydroaltersolanol A (58, Figure 6, Table 1), and five new alterporriol-type anthranoid dimers, named alterporriols N−R (59–63, Figure 6), were isolated from the culture broth and the mycelia of Alternaria sp. ZJ-2008003.
Figure 6.
Bioactive anthraquinones and analogues produced by Alternaria sp.
The fungus also produced seven known analogues as tetrahydroaltersolanol B, altersolanol B, altersolanol C, altersolanol L, ampelanol, macrosporin (8, Figure 2) and alterporriol C. The fungus was isolated from Sarcophyton sp. soft coral collected from the South China Sea. All the compounds were assayed against the porcine reproductive and respiratory syndrome virus (PRRSV) and 54 and 62 showed antiviral activity with IC50 values of 65 and 39 μM, respectively. Compound 61 exhibited cytotoxic activity against PC-3 and HCT-116 cell lines, with IC50 values of 6.4 and 8.6 μM, respectively [47].
Holoroquinone (64, Figure 7, Table 1), is an anthraquinone isolated from a marine-derived fungus Halorosellinia sp., which showed antitumor activity. Its biosynthesis was elucidated by incorporation of [2-13C]malonate and [1,2,3-13C]malonate followed by 13C-NMR investigation [48].
Figure 7.
Bioactive anthraquinones and analogues produced by Halorosellinia sp., Torrubiella sp. BBC 28517, Hypocreales sp. MSX 17022, Penicillium citrinum PSU-F51 and Stemphylium sp. 33231.
Two new dimeric anthraquinones with a rare chemical skeleton, named torrubiellins A and B (65 and 66, Figure 7, Table 1), were isolated from Torrubiella sp. BCC 28517 (family Clavicipitaceae) belonging to a genus of fungus that attacks spiders, scale-insects, and hoppers. Torrubiellin B (66) exhibited a broad range of biological activities including strong antimalarial (Plasmodium falciparum), antifungal (Candida albicans), antibacterial (Bacillus cereus) activities, and cytotoxicity to cancer cell lines. Its biological activity was always higher than that of torrubiellin A (65) [49].
Two new xanthone–anthraquinone heterodimers, named acremoxanthones C and D (67 and 68, Figure 7, Table 1), were isolated from an unidentified fungus of the Hypocreales order (MSX 17022). The fungus also produced the close and already known acremonidins A and C, benzophenone, and moniliphenone. All the metabolites showed moderate cytotoxic activity in vitro. In addition, acremoxanthone D (68), and acremonidins A and C exhibited moderate 20S proteasome inhibitory activity [50].
Two new anthraquinone citrinin derivatives, named penicillanthranins A and B (69 and 70, Figure 7, Table 1), were isolated together with 3R,4S-dihydrocitrinin from the mycelium extract of the sea fan-derived fungus Penicillium citrinum PSU-F51. Penicillanthranin A (69) showed moderate antibacterial activity against Staphylococcus aureus ATCC25923 with MIC values of 16 μg/mL and methicillin-resistant S. aureus SK1. Compounds 69 also exhibited mild cytotoxicity toward KB cells with IC50 values of 30 μg/mL [51]. Two new benzopyranones and one isochroman together with several known compounds as methyl 2-(2-acetyl-3,5-dihydroxy-4,6-dimethylphenyl)acetate, coniochaetone A, decarboxydihydrocitrinin, 1-acetonyl-7-carboxyl-6,8-dihydroxy-3,4,5-trimethylisochroman 6,8-dihydroxy-3,4,5,7-tetramethyl-3,4-dihydroisocoumarin, methyl 8-hydroxy-6-methylxanthone-1-carboxylate, sydowinin A, pinselin, conioxanthone A, chrysophanol, emodin, and ω-hydroxyemodin were isolated from the culture filtrates of the same fungus [51].
Four new anthraquinone derivatives, named auxarthrol C, macrosporin 2-O-(6′-acetyl)-α-d-glucopyranoside (71 and 72, Figure 7), 2-O-acetylaltersolanol B, and 2-O-acetylaltersolanol L (73 and 74, Figure 8), and four new alterporriol-type anthranoid dimers, named alterporriols T–W (75–78, Figure 8), were isolated along with 17 analogues from the rice culture of Stemphylium sp. 33,231 obtained from the mangrove Bruguiera sexangula var. rhynchopetala collected from the South China Sea [52]. The already known compounds were identified as dihydroaltersolanol A, macrosporin, macrosporin-7-O-sulfate, altersolanols A–C and L, ampelanol, tetrahydroaltersolanol B and alterporriols A–E, N, and Q. Compounds 78, showed only weak antibacterial activity against E. coli, S. aureus, and B. subtilis, suggesting that anthraquinone derivatives showed better antibacterial activities than anthraquinone dimers in these assays. Compounds 78 also showed a moderate lethality, with an LD50 value of 10 μM when tested on brine shrimp lethality using Artemia salina [52].
Figure 8.
Bioactive antharaquinones and analogues produced by Stemphylium sp. 33231, Penicillium purpurogenum, the endophytic fungus ZSUH-36 and A. versicolor.
Eight known anthraquinone derivatives such as 6,8,10-tri-O-methyl averantin, 6,8-di-O-methyl averufnin, 6,8-di-O-methyl averufanin (79–81, Figure 8, Table 1) aversin, 1,3-dihydroxy-6,8-dimethoxy-9,10-anthraquinone, 6,8-di-O-methylnidurufin, 6,8-di-O-methyl versiconol and 5-methyoxysterigmatocystin (82–86, Figure 9, Table 1) were isolated from the extracts of Penicillium purpurogenum together with (S)-ornidazole. Only compounds 79 and 86 exhibited strong toxicity against brine shrimp (Artemia salina) at 10 mM, with lethality rates of 100% comparable to the positive control toosendanin. Compounds 79, 82, and 85 moderately inhibited the growth of Botrytis cinerea. Compound 82 showed moderate antifungal activity against Gibberella saubinettii, while compounds 84, 85 and (S)-ornidazole exhibited phytotoxicity on radish seedlings at 100 mM [53]. 6,8,1′-Tri-O-methyl averantin (79), 6,8-di-O-methyl averufanin (80), and 6,8-di-O-methyl averufin (81), together with three known anthraquinones 1-O-methyl averantin, averufin (9), and versicolorin C were also produced by the endophytic fungus ZSUH-36 isolated from a mangrove collected from the South China Sea. At that time, only the unambiguous structure of 79, being a new anthraquinone, was determined by advanced NMR spectra while no activity was described [54]. Previously, compounds 82 and 85, together with the two xanthones 5-methoxysterigmatocystin and sterigmatocystin (86 and 87, Figure 9, Table 1), had been isolated from the same fungus and only the unambiguous structure of compound 85, which at that time was a new anthraquinone, was determined by advanced NMR spectra [55]. Compounds 80–82 and 84 were previously isolated from the culture of Aspergillus versicolor, an endophytic fungus obtained from the marine brown alga Sargassum thunbergii.
Figure 9.
Bioactive anthraquinones and analogues produced by P. purpurogenum, the endophytic fungus ZSUH-36, A. versicolor, Aspergillus sp. YL-6, Gliocladium sp. T31 and Engyodontium album.
Asperversin, A, 9ξ-O-2(2,3-dimethylbut-3-brevianamide Q, brevianamide K, brevianamide M, 6,8-5α,8α-epidioxyergosta-6,22-dien-3β-ol, ergosta-7,22-diene3β,5α,6β-triol, and 6β-methoxyergosta-7,22-diene-3β,5α-diol were isolated from the same fungus. Compound 81 exhibited antibacterial activity against Escherichia coli and S. aureus, and lethality against brine shrimp (Artemia salina) with an LC50 value of 0.5 μg/mL [56].
Two anthraquinones identified as questin and isorhodoptilometrin (88 and 89, Figure 9, Table 1) were produced together with chaetominine, (+)-alantrypinone, and 4-hydroxybenzaldehyde from the endophytic fungus Aspergillus sp. YL-6, isolated from the allelopathic plant Pleioblastus amarus. When all the metabolites were tested on wheat (Triticum aestivum) and radish (Raphanus sativus) at concentrations of 10 and 20 ppm, they inhibited the germination and growth of the two plant seeds completely. Among them (+)-alantrypinone, an indole-3-acetic acid (IAA), showed the best effects similar to that induced by glyphosate, a broad-spectrum systemic herbicide. Furthermore, questin (88), inhibited shoot and root elongation of wheat, always similar to glyphosate [57]. Questin was previously isolated together with another three anthraquinones, identified as fallacinol, citreorosein, and questinol (90–92, Figure 9, Table 1), and protocatechuic acid, (+)-catechin, 2,5-dimethyl-7-hydroxy chromone, 7-hydroxy-4-methoxy-5-methylcoumarin, torachrysone-8-O-d-glucoside, and 2-methoxy-6-acetyl-7-methyljuglone from the dried roots of Polygonum cuspidatum, a plant extensively used in Chinese and Japanese folk medicine [58]. Isorhodoptilometrin (89) was previously isolated together with secalonic acid D, emodin (21), and citreorosein (91), from a marine lichen-derived fungus Gliocladium sp. T31 with secalinic acid D showing strong cytotoxic activity against human myeloid leukemia K562 cell line [59].
Engyodontochones A (93, Figure 9, Table 1) and B–F (94–97, Figure 10, Table 1) together with two known polyketides as a betacolin-like metabolite (99, Figure 9, Table 1) and JBIR-99 (100, Figure 10, Table 1), all belonging to the anthraquinone–xanthone subgroup of polyketides, were isolated from mycelia and culture broth of Engyodontium album strain LF069. E. album, was found in soil extreme environments, plant debris, and in indoor environments on paper, textile, jute, and painted walls. It is a pathogenic fungus, inducing serious human diseases. Engyodontochones A–E (93, 94–96) and betacolin-like metabolite (99) exhibited inhibitory activity, 10-fold stronger than chloramphenicol, against methicillin resistant S. aureus (MRSA) [60].
Figure 10.
Bioactive anthraquinones and analogues produced by Engyodontium album, Nigrospora sp. and Aspergillus tritici.
Seven anthraquinone derivatives identified as 1,2,8-trihydroxyanthraquinone, 1,3,8-trihydroxyanthraquinone, 1,3,6-trihydroxy-8-methylanthraquinone, rheoemodin, aloesaponarin II, isozyganein, 1-acetyl-4,5-dihydroxy-anthraquinone (101–107, Figure 10, Table 1) were isolated together with cis-4-hydroxyscytalone, and cerebroside B from the culture filtrates of the endophytic fungus Nigrospora sp. cis-4-Hydroxyscytalone showed strong antibacterial activities against E. coli and B. subtilis with MIC values of 64 and 128 μg/mL, respectively. 1,3,8-Trihydroxyanthraquinone, 1,3,6-trihydroxy-8-methylanthraquinone, and aloesaponarin II (102, 103, and 105) exhibited inhibitory activity against P. oryzae with MIC values of 128 μg/mL, while 1,3,8-trihydroxyanthraquinone (102) showed moderate antifungal activity against C. albicans with MIC value of 128 μg/mL. Rheoemodin (104) exhibited weak antimicrobial activity only against E. coli with MIC values of 256 μg/mL [61]. Isozyganein (106) was also previously synthesized and showed weak antioxidative activity [62].
Aspetritones A and B (108 and 109, Figure 10, Table 1) were isolated from the culture of the coral-derived fungus Aspergillus tritici SP2-8-1, together with 4-methyl-candidusin A and fifteen known metabolites belonging to different classes of natural compounds as prenylcandidusin, candidusin, and terphenyllin derivatives and anthraquinones. Bostrocyn (110, Figure 10, Table 1) and other four anthraquinones (111–114, Figure 10, Table 1) were isolated. Aspetritone A (108) showed the most significant activity against methicillin-resistant strains of S. aureus in respect to that of the positive control chloramphenicol and exhibited strong cytotoxicity against human cancer cell lines HeLa, A549, and Hep G2 [63].
A new anthraquinone glycoside derivative namely, 1-O-methyl-6-O-(α-d-ribofuranosyl)-emodin (115, Figure 11, Table 1), was produced together with two new resorcinol glycoside derivatives as resorcinol 2-butyl-5-pentyl-4-C-6-deoxy-β-d-gulopyranoside and resorcinol 2-butyl-5-pentyl-4-C-α-l-rhamnoside, named stemphols C and D, and eight already known compounds from the culture filtrates of the endophytic fungus Gaeumannomyces sp. isolated from the rhizome of a halophyte, Phragmites communis, in Suncheon bay, South Korea [64]. In particular, among the known compounds, 1-O-methylemodin (116, Figure 11, Table 1) was also identified. Compound 116 was first isolated together with 5-chloro-6,8-dihydroxy-1-methoxy-3-methylanthraquinone, 7-chloro-6,8-dihydroxy-1-methoxy-3-methylanthraquinone, 5-chloro-6,8,10-trihydroxy-1-methoxy-3-methyl-9(10H) anthracenone and 5-chloro-8,10-dihydroxy-l,6-dimethoxy-3-metbyl-9(10H)-anthracenone (117–120, Figure 11, Table 1), from Phialophora alba, a fungus that might protect the aspen from attack by other fungi [65]. Compounds 115 as well as the two glycosyl derivatives of resorcinol showed anti-inflammatory properties while 1-O-methylemodin (116) reduced NO production by LPS-treated cells by 43% and 31%, respectively, without inducing cell death [64].
Figure 11.
Bioactive anthraquinones and analogues produced by Gaeumannomyces sp., Phialophora alba, Eurotium rubrum, A. glaucus, Coniothyrium sp., Penicillium sp. OUCMDZ-4736, Fusarium solani and Paraconiothyrium sp.
Compound 115 also inhibited the growth of the tree decaying fungus Phellinus tremulae [66], the secretion of IL-625 [67], and of protein tyrosine phosphatase 1B [68].
Rubrumol (121, Figure 11, Table 1) was isolated together with four known analogues as emodin and catenarin (21 and 24), rubrocristin, 2-methyleurotinone, and conyothyrinone A (122–124, Figure 11, Table 1) from the solid culture of Eurotium rubrum, a fungus obtained from the salt-tolerance wild plant Suaeda salsa L. which was collected from ‘BoHai’ seaside. Among all the compounds, only 121 showed activity when tested on Topo I to relax supercoiled pBR322 DNA and it did not show cytotoxic activities against A549, MDA-MB-231, PANC-1, and HepG2 human cancer cell [69]. Rubrocristin (122) was previously isolated from the mycelia of Aspergillus glaucus together with physcion (23) emodin, and catenarin (21 and 24), questin (88), erythroglaucin, physcion-9-anthrone, viocristin, and isoviocristin (125–128, Figure 11, Table 1). Compounds 21, 24, 127, and 128 showed antibacterial activity with minimal inhibitory concentrations ranging from 1–10 pg/mL, while compounds 21, 24, and 127 inhibited the incorporation of uridine and thymidine in Ehrlich ascites carcinoma cells while 24 and to a lesser extent compound 21 also inhibited in vitro DNA-dependent RNA polymerase from Escherichia coli [70]. Conyothyrinone A (124, Figure 11, Table 1) was also isolated from the culture of Coniothyrium sp. together with conyothyrinones B–D (129–131, Figure 11, Table 1) and the already known ones pachybasin and phomarin (51 and 52) as well as 1,7-dihydroxy-3-methyl-9,10-anthraquinone and 1-hydroxy-3-hydroxymethyl-9,10-anthraquinone (132 and 133, Figure 11, Table 1), an endophytic fungus isolated from Salsola oppostifolia from Gomera in the Canary islands. All these metabolites were tested for their antifungal, antibacterial, and algicidal properties. Compound 132 showing a strong antibacterial activity against Bacillus magaterium and E. coli. Phomarin and conyothyryrinone A (52 and 124) exhibited strong antifungal activity against Mycrobotryum violaceum and B. cinerea, while pachybasin (51), which is the main metabolite, showed weak activity against B. megaterium [71].
Three new anthraquinones, identified as (-)-2′R-1-hydroxyisorhodoptilometrin, methyl 3,4,8-trihydroxy-6-methyl-9-oxo-9H-xanthene-1-carboxylate, and methyl 6,8-dihydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylate (134–136, Figure 11, Table 1) were isolated from the culture filtrates, Penicillium sp. OUCMDZ-4736, on the effect of acid stress conditions. The fungus was isolated from the sediment around the roots of the mangrove (Acanthus ilicifolius). The new anthraquinones were isolated together with ten already known compounds including asterric acid, parietinic acid, endocrocin, and monochlorsulochrin [72]. Compound 134, on comparison with the control lamivudine showed a stronger anti-hepatitis B virus inhibiting HBsAg and HBeAg secretion from HepG2.2.15 cells [73].
Danthron (137, Figure 11, Table 1), characterized as 1,8-dihydroxyanthraquinone, was produced as the main bioactive metabolite by the fungal endophyte Paraconiothyrium sp. isolated from Zingiber officinale. Compound 137 showed antifungal activity against clinical pathogens and against the phytopathogen Pythium myriotylum, which causes Pythium rot in ginger [74]. Danthron, also called as chrysazin, was used as a stimulant laxative in some countries and showed antibacterial, antifungal [75], and anticancer activities [76].
An aza-anthraquinone identified as bostrycoidin (138, Figure 11, Table 1) was produced together with anhydrofusarubin, fusarubin, 3-deoxyfusarubin, ergosterol, 3,5,9-trihydroxyergosta-7,22-diene-6-one, and 4-hydroxybenzaldehyde from large scale cultivation of the endophytic fungus Fusarium solani isolated from Cassia alata Linn. growing in Bangladesh.
The crude organic extract of the fungal culture filtrates showed cytotoxicity, using “Brine Shrimp Lethality Bioassay”, antimicrobial and antioxidant activity. Among the isolated metabolites, compound 138 appeared to be the most potent anticancer and antimicrobial metabolite [77].
Three new phytotoxic anthraquinones, named lentiquinones A–C (139–141, Figure 12, Table 1) were isolated from the culture filtrates of Ascochyta lentis inducing Ascochyta blight on lentil (Lens culinaris Medik.) [78]. Another new anthraquinone, named lentisone (142 Figure 12, Table 1), together with the known pachybasin (51), tyrosol, and pseurotin A, was previously isolated from the same fungus [79]. Pachybasin (51) was also isolated, as the main metabolite, from the fungal mycelium of the same fungus together with other three known anthraquinones as ω-hydroxypachybasin (143, Figure 12, Table 1), 1,7-dihydroxy-3-methylanthracene-9,10-dione (144, Figure 12, Table 1), and phomarin (52) [78]. Tested by leaf-puncture on host and non-host plants, the three new anthraquinones (139–141) and lentisone (142) caused severe necrosis, with lentiquinone A being the most active. Compound 139 proved to be particularly active on cress (Lepidium sativum), in inhibiting root elongation. Furthermore, all the compounds reduced the content of chlorophyll in Lemna minor, with 1,7-dihyroxy-3-methylanthracene-9,10-dione (144) being the most active. The lentiquinones A–C and lenstisone had antibiotic properties [78].
Figure 12.
Bioactive anthraquinones and analogues produced by Fusarium solani, Ascochyta lentis, A. versicolor and Aspergillus sp. SCSIO F063.
Two anthraquinone dimers (145 and 146, Figure 12, Table 1) were produced together with another three known anthraquinones as 1′-O-methylaverantin and averantin (147 and 148, Figure 12, Table 1), averythrin (7), and two xanthones as stergmatocystin and variecoxanthone from the marine-derived fungus Aspergillus versicolor. Compounds 145 and 146, showed selective antibacterial activity against S. aureus, while stergmatocystin exhibited moderate cytotoxicity against human cancer cell lines [15]. Averantin was also isolated from the marine-derived fungus Aspergillus sp. SCSIO F063 together with its seven related chlorinated anthraquinones as (1′S)-7-chloroaverantin, (1′S)-6-O-methyl-7-chloroaverantin, (1′S)-1′-O-methyl-7-chloroaverantin, (1′S)-6,1′-O,O-dimethyl-7-chloroaverantin, (1′S)-7-chloroaverantin-1′-butyl ether, 7-chloroaverythrin, and 6-O-methyl-7-chloroaverythrin (149–155, Figure 13, Table 1). Five known analogues, identified as 1′-O-methylaverantin, 6-O-methylaverantin, averantin-1′-butyl ether, and averythrin (7) were also isolated when the fungus was grown on sea salt-containing potato dextrose broth. When sodium bromide was added to the culture medium also two new brominated anthraquinones as (1′S)-6,1′-O,O-dimethyl-7-bromoaverantin and (1′S)-6-O-methyl-7-bromoaverantinone (156 and 157, Figure 13, Table 1) and a nonhalogenated anthraquinone, identified as (1′S)-6,1′-O,O-dimethylaverantin (158, Figure 13, Table 1) were extracted from fungal mycelia. Among all the compounds isolated only 6-O-methyl-7-chloroaveratin (155) displayed inhibition activity against three human tumor cell lines, SF-268, MCF-7, and NCI-H460, with IC50 values of 7.11, 6.64, and 7.42 μM, respectively [47].
Figure 13.
Bioactive anthraquinones and analogues produced by Aspergillus sp. SCSIO F063, Alternaria tenuissima, Talaromyces funiculosus, Neofusicoccum luteum and Rubia tinctorum.
A hydroanthraquinone with a hexacyclic spiro-fused ring system and two new anthraquinones with a 4,5-disubstituted butylaminolate unit, named anthrininones A–C (159–161, Figure 13, Table 1) were isolated from the deep-sea derived fungus Alternaria tenuissima DFFSCS013. They were isolated together with six known analogues including 6-O-methylalaternin (162, Figure 13, Table 1), 10,11- dihydroaltersolanol A, altersolanol L, ampelanol, (3R)-1-deoxyaustrocortilutein and altersolanol B. Compounds 159–162 showed strong inhibition activity against indoleamine 2,3-dioxygenase 1 (IDO1), and compounds 160–162 also had selective inhibition activity against different protein tyrosine phosphatase, while compound 159 stimulated intracellular calcium levels at a concentration of 10 μM [81].
Funiculosone (163, Figure 13, Table 1), a new substituted dihydroxanthene-1,9-dione, was isolated together with its two known analogues mangrovamide J and ravenelin (164–165, Figure 13, Table 1) from the culture filtrates of Talaromyces funiculosus (Trichocomaceae) an endolichenic fungus obtained from lichen thallus of Diorygma hieroglyphicum in India [82]. When assayed against E. coli and S. aureus, all the compounds displayed antibacterial activity with an IC50 range 23–104 μg/mL. Compound 163 also showed anticandidal activity against Candida albicans with an IC50 35 μg/mL [82].
A new hexasubstituted anthraquinone, named neoanthraquinone (166, Figure 13, Table 1) was isolated from Neofusicoccum luteum, the causal agent of Botryosphaeria dieback in Australia. N. luteum produced also a new disubstituted furo-α-pyrone and a trisubstituted oxepi-2(7H)-one, named luteopyroxin and luteoxepinone respectively, together with the known (±)-nigrosporione, tyrosol, (R)-(−)-mellein and (3R,4S)-(−)- and (3R,4R)-(−)-4-hydroxymellein. Compound 166 caused severe shriveling and withering when assayed on grapevine leaves, while the other metabolites showed different degrees of toxicity [83].
3. Industrial Application of Anthraquinones
Since 1869 with the determination of the structure of alizarin (167, Figure 13, Table 1), a yellow anthraquinone, the main industrial application of this anthraquinone was its use as a dye in textile manufacturing [85]. Compound 167 was isolated for the first time from Rubia tinctorum [84]. Thus, over a span of 20 years many analogues with different functionalities were also prepared by synthesis to obtain different dyes such as red, blue, and green mordant. Successively, the first acidic anthraquinone dye used to color wool without pretreatment with mordants was reported. At the beginning of 1900 the sulfonation and nitration of anthraquinone opened up a new era for anthraquinone based dyes.
A new phase in this development occurred with the introduction of synthetic fibers, such as polyester, polyamide, and polyacrylonitrile fibers, with the substitution of anthraquinones with other dyes. The use of acid anthraquinone dyes increased with the discovery of the first fiber-reactive dyes. At the same time, the utilization of natural substances instead of synthetic ones, increased worldwide. This satisfy the request of environmentally friendly sustainable technologies. As reported in Section 1 fungi are a significant source of pigments as several genera can produce pigments in good amounts identified as anthraquinones or analogues. The production of anthraquinones by fungal fermentation had been developed for rapid and easy growth to produce pigments useful in various industrial applications [86]. The natural anthraquinones, as well as other natural pigments, have noteworthy less toxic effects than the synthetic dyes and are easily degradable avoiding the high environmental pollution. Thus, these anthraquinone based dyes are used in medical, textile coloring, food coloring, and cosmetic industries [84,86].
On this basis also plants have been largely used as a source of natural colored anthraquinones. In fact, screening of dyeing plants was carried out for their widespread use in previous centuries. Colorimetric analysis showed that the principal color was yellow-orange shades and could be attributed to flavonoids while the red colors were due to anthraquinones. Colors from plants that contain anthocyanins varied from blue-violet through to red. The nature of the support fibers (wool or cotton) plays an important role in the perceived colors [87].
At the same time several anthraquinone based dyes were synthesized for industrial applications as nitro derivatives useful also as dye intermediates. The reactions used were nitration methods for the preparation of 1- and 2-nitroanthraquinones and 1,5-, 1,8-, 1,6-, 1,7-, 1,8-, 2,6-, and 2,7-dinitroanthraquinone. These were also used to obtain their reduced analogues such as 1-amino-anthraquinone and 1,5-diaminoanthraquinones, both useful to produce vat dyes. Other methods were the preparation of 1-SO3H and 1-MeNH derivatives of anthraquinones useful for manufacturing of dyes for wool, acetate rayon, and polyamide fibers. Another method enables the preparation of leuco-1,4,5,8-tetrahydroxyanthraquinone useful for synthesis of acid and disperse dyes [88].
Recently water-repellent, self-cleaning and stain resistant textiles were obtained by developing anthraquinone reactive dyes which were covalently grafted onto cotton fabric surfaces obtaining bright colors with good wash-fastness properties and giving rise to breathable superhydrophobic textiles with self-cleaning properties [89].
The large number of textile dyes required a method for their classification which was based on the functional groups attached to the typical anthraquinone carbon skeleton. Thus, there are: anthraquinone, azo, phthalocyanine, sulfur, indigo, nitro, nitroso anthraquinone derivatives etc. taking into account their chemical structures. Another classification was based on the method of applying these dyes on an industrial scale, grouped as disperse, direct, acid, reactive, basic, vat dyes etc. [90].
The intensity of research focused on natural compounds has been growing over the past few decades. Anthraquinones have been most studied in China producing several publications which report different advanced extraction methods, analytical techniques, and industrial applications. These publications also describe the most used plants for anthraquinone content as Polygonaceae, Rubiaceae, and Fabaceae and report the best known anthraquinones: rhein aloe emodin, emodin, physcion, chrysophanol which are responsible for their numerous biological properties. Furthermore, the use of natural anthraquinones for industrial applications, has been described as an alternative to synthetic dyes to avoid some unwanted side effects [9].
However, the environmental contamination by wastewater containing dyes is today a severe problem to solve. The application of advanced oxidation processes (AOPs) to industrial wastewater has increased as well as an integrated approach for their biological and chemical treatment. The toxicity of the detergents and the dye have been determined in terms of effective concentration EC50 using mixed cultures of activated sludge as well as a pure culture of luminescent bacteria Vibrio fischeri NRRLB-11177. However, the dye was not degraded without AOP pretreatment, therefore the degree of its removal (decolorization) by the AOPs is an important preliminary stage of bio-sorption on activated sludge [91].
4. Conclusions
The sources, structures, and the biological activities of fungal bioactive anthraquinones were reported starting from 1966 to the present day. In the introduction the previous review published on this topic was also cited which did not however treat the topic extensively. The anthraquinones were chronologically described and in some cases their isolation and biological activity was investigated in depth. Furthermore, their industrial application in different fields, essentially as natural dyes, was also reported focusing on the comparison between natural and synthetic anthraquinone based dyes, their chemical derivatization and classification, and the advanced methods used in the treatment of the relative industrial wastewaters to avoid severe negative environmental pollution.
Acknowledgments
A.E. is associate to the Istituto di Chimica Biomolecolare, CNR, Pozzuoli, Italy.
Author Contributions
The authors equally contributed to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This review, covering the literature from 1966 to the present (2020), concerns fungal anthraquinones and their biological activity including phytotoxic, antibacterial, antiviral, anticancer, algicide, antifungal, enzyme inhibiting, immunostimulant, antiplatelet aggregation, and antiplasmodium activities. The review also covers some practical industrial applications of anthraquinones.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Turner W.B., Aldridge D.C. Fungal Metabolites II. Academic Press; London, UK: 1983. [Google Scholar]
- 2.Osbourn A.E., Lanzotti V. Plant-Derived Products. Springer; Dordrecht, Germany: 2009. [Google Scholar]
- 3.Dewick P.M. Medicinanal Natural Products—A Biosynthetic Approach. Wiley and Sons Ltd.; Chicester, UK: 2009. [Google Scholar]
- 4.Locatelli M. Anthraquinones: Analytical techniques as a novel tool to investigate on the triggering of biological targets. Curr. Drug Targets. 2011;12:366–380. doi: 10.2174/138945011794815338. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Liang C., Chen Q., Yang Z. Rhein: A potential biological therapeutic drug for intervertebral disc degeneration. Med. Hypotheses. 2011;77:1105–1107. doi: 10.1016/j.mehy.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Mizushina Y., Nishiumi S., Nishida M., Yoshida H., Azuma T., Yoshida M. Inhibition of repair-related DNA polymerases by vitamin Ks, their related quinone derivatives and associated inflammatory activity. Int. J. Oncol. 2013;42:793–802. doi: 10.3892/ijo.2013.1771. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W., Bounda G.A., Yu F. Pharmacological potential action of rhein and its diverse signal transduction-a systematic review. World J. Pharm. Res. 2014;3:3599–3626. [Google Scholar]
- 8.Sultan S., Ghani N.A., Shah S.A.A., Ismail N.H., Noor M.Z., Naz H. Microbial transformation of bioactive anthraquinones—A review. Biosci. Biotechnol. Res. Asia. 2013;10:577–582. doi: 10.13005/bbra/1167. [DOI] [Google Scholar]
- 9.Duval J., Pecher V., Poujol M., Lesellier E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crops Prod. 2016;94:812–833. doi: 10.1016/j.indcrop.2016.09.056. [DOI] [Google Scholar]
- 10.Gessler N.N., Egorova A.S., Belozerskaya T.A. Fungal anthraquinones. Appl. Biochem. Microbiol. 2013;49:85–99. doi: 10.1134/S000368381302004X. [DOI] [Google Scholar]
- 11.Fouillaud M., Venkatachalam M., Girard-Valenciennes E., Caro Y., Dufossé L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs. 2016;14:64. doi: 10.3390/md14040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai M., Gade A., Zimowska B., Ingle A.P., Ingle P. Marine-derived Phoma-the gold mine of bioactive compounds. Appl. Microbiol. Biotechnol. 2018;102:9053–9066. doi: 10.1007/s00253-018-9329-2. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher R., Hodges R. The chemistry of dothistromin, a difuronanthraquinone from Dothistroma pini. Aust. J. Chem. 1972;25:2399–2407. doi: 10.1071/CH9722399. [DOI] [Google Scholar]
- 14.Danks A.V., Hodges R. Polyhydroxyanthraquinones from Dothistroma pini. Aust. J. Chem. 1974;27:1603–1606. doi: 10.1071/CH9741603. [DOI] [Google Scholar]
- 15.Li J.L., Jiang X., Liu X., He C., Di Y., Lu S., Huang H., Linc B., Wangd D., Fan B. Antibacterial anthraquinone dimers from marine derived fungus Aspergillus sp. Fitoterapia. 2019;133:1–4. doi: 10.1016/j.fitote.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Nakaishima S. Studies on the metabolites of phytotoxic fungi. I. Isolation of macrosporin and 6-methylxanthopurpurin 3-methyl ether from Alternaria bataticola Ikata ex Yamamoto. Chem. Pharm. Bull. 1973;21:2083–2085. [Google Scholar]
- 17.Evidente A., Rodeva R., Andolfi A., Stoyanova Z., Perrone C., Motta A. Phytotoxic polyketides produced by Phomopsis foeniculi, a strain isolated from diseased Bulgarian fennel. Eur. J. Plant Pathol. 2011;130:173–182. doi: 10.1007/s10658-011-9743-0. [DOI] [Google Scholar]
- 18.Barash I., Karr A.L., Strobel G.A. Isolation and characterization of stemphylin, a chromone glucoside from Stemphylium botryosum. Plant Physiol. 1975;55:646–651. doi: 10.1104/pp.55.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barash I., Netzer D., Nachmias A., Strobel G.A. Differential effect of phytotoxins produced by Stemphylium botryosum on susceptible and resistant lettuce cultivars. Phytoparasitica. 1978;6:95–98. doi: 10.1007/BF02980389. [DOI] [Google Scholar]
- 20.Assante G., Nasini G. Identity of the phytotoxin stemphylin from Stemphylium botryosum with altersolanol A. Phytochemistry. 1987;26:703–705. doi: 10.1016/S0031-9422(00)84768-8. [DOI] [Google Scholar]
- 21.Sakamura S., Ichihara A., Yoshihara T. In: Biologically Active Natural Products. Cutler H.G., editor. American Chemical Society; Washington, DC, USA: 1988. ACS Symposium Series. [Google Scholar]
- 22.Sparapano L., Mairota P., Lerario P. In: Phytotoxins and Plant Pathogenesis. Graniti A., Durbin R.D., Ballio A., editors. Springer; Berlin/Heidelberg, Germany: 1989. [Google Scholar]
- 23.Corsaro M.M., De Castro C., Evidente A., Lanzetta R., Molinaro A., Parrilli M., Sparapano L. Phytotoxic extracellular polysaccharide fractions from Cryphonectria parasitica (Murr.) Barr strains. Carbohydr. Polym. 1998;37:167–172. doi: 10.1016/S0144-8617(98)00050-2. [DOI] [Google Scholar]
- 24.Calhoun L.A., Findlay J.A., Miller J.D., Whitney N.J. Metabolites toxic to spruce budworm from balsam fir needle endophytes. Mycol. Res. 1992;96:281–286. doi: 10.1016/S0953-7562(09)80939-8. [DOI] [Google Scholar]
- 25.Frisvad J.C., Rank C., Nielsen K.F., Larsen T.O. Metabolomics of Aspergillus fumigatus. Med. Mycol. 2009;47:S53–S71. doi: 10.1080/13693780802307720. [DOI] [PubMed] [Google Scholar]
- 26.Wijesekara I., Zhang C., Van Ta Q., Vo T.S., Li Y.X., Kim S.K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol. Res. 2014;169:255–261. doi: 10.1016/j.micres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Thomson R.H. Naturally Occurring Quinones. Academic Press; London, UK: New York, NY, USA: 1971. [Google Scholar]
- 28.Engström K., Brishammar S., Svensson C., Bengtsson M., Andersson R. Anthraquinones from some Drechslera species and Bipolaris sorokiniana. Mycol. Res. 1993;97:381–384. doi: 10.1016/S0953-7562(09)81142-8. [DOI] [Google Scholar]
- 29.Bouras N., Strelkov S.E. The anthraquinone catenarin is phytotoxic and produced in leaves and kernels of wheat infected by Pyrenophora tritici-repentis. Physiol. Mol. Plant Pathol. 2008;72:87–95. doi: 10.1016/j.pmpp.2008.06.001. [DOI] [Google Scholar]
- 30.Brady S.F., Wagenaar M.M., Singh M.P., Janso J.E., Clardy J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000;2:4043–4046. doi: 10.1021/ol006680s. [DOI] [PubMed] [Google Scholar]
- 31.Brady S.F., Singh M.P., Janso J.E., Clardy J. Cytoskyrins A and B, new BIA active bisanthraquinones isolated from an endophytic fungus. Org. Lett. 2000;2:4047–4049. doi: 10.1021/ol006681k. [DOI] [PubMed] [Google Scholar]
- 32.Elespuru R.K., Yarmolinsky M.B. A colorimetric assay of lysogenic induction designed for screening potential carcinogenic and carcinostatic agents. Environ. Mutagen. 1979;1:65–78. doi: 10.1002/em.2860010113. [DOI] [PubMed] [Google Scholar]
- 33.Elespuru R.K. In: Environmental Physiology and Medicine, Short-Term Tests for Chemical Carcinogens. Stich H., San C., editors. Springer; New York, NY, USA: 1981. pp. 1–11. [Google Scholar]
- 34.Elespuru R.K., Moore S.G. Micro-BIA, a colorimetric microtiter assay of λ prophage induction. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1986;164:31–40. doi: 10.1016/0165-1161(86)90039-7. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M., Ohra J., Tsujtno Y., Fujimori T., Ago H., Tsuge H.Z. Dendryol A, B, C, and D, four new compounds produced by a weed pathogenic fungus Dendryphiella sp. Naturforsch. C. 1995;50:751–756. doi: 10.1515/znc-1995-11-1203. [DOI] [Google Scholar]
- 36.Imaizumi S., Tokumasu S., Nitta N., Fujimori T., Yamada M. A new disease of paddy weed Eleocharis kuroguwai caused by Dendryphiella sp. Ann. Phytopath. Soc. Jpn. 1992;58:158. [Google Scholar]
- 37.Huss H., Mayerhofer H., Wetschnig W. Ophiocladium hordei CAV. (Fungi imperfecti), ein für Östereich neuer parasitischer Pilz der Gerste. Der Pflanzenarzt. 1987;40:11–13. [Google Scholar]
- 38.Miethbauer S., Haase S., Schmidtke K.U., Günther W., Heiser I., Liebermann B. Biosynthesis of photodynamically active rubellins and structure elucidation of new anthraquinone derivatives produced by Ramularia collo-cygni. Phytochemistry. 2006;67:1206–1213. doi: 10.1016/j.phytochem.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Miethbauer S., Gaube F., Möllmann U., Dahse H.M., Schmidtke M., Gareis M., Pickhardt M., Liebermann B. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungus Ramularia collo-cygni. Planta Med. 2009;75:1523–1525. doi: 10.1055/s-0029-1185835. [DOI] [PubMed] [Google Scholar]
- 40.Miethbauer S., Gunther W., Schmidtke K.U., Heiser I., Grafe S., Gitter B., Liebermann B. Uredinorubellins and caeruleoramularin, photodynamically active anthraquinone derivatives produced by two species of the genus Ramularia. J. Nat. Prod. 2008;71:1371–1375. doi: 10.1021/np800138y. [DOI] [PubMed] [Google Scholar]
- 41.Vinale F., Ghisalberti E.L., Sivasithamparam K., Marra R., Ritieni A., Ferracane R., Woo S., Lorito M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett. Appl. Microbiol. 2009;48:705–711. doi: 10.1111/j.1472-765X.2009.02599.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y.M., Li H., Hong J., Cho H.Y., Bae K.S., Kim M.A., Kim D.K., Jung J.H. Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 2010;33:231–235. doi: 10.1007/s12272-010-0207-4. [DOI] [PubMed] [Google Scholar]
- 43.Hawas U.W., El-Beih A.A., El-Halawany A.M. Bioactive anthraquinones from endophytic fungus Aspergillus versicolor isolated from red sea algae. Arch. Pharm. Res. 2012;35:1749–1756. doi: 10.1007/s12272-012-1006-x. [DOI] [PubMed] [Google Scholar]
- 44.Cimmino A., Masi M., Evidente M., Superchi S., Evidente A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015;32:1629–1653. doi: 10.1039/C5NP00081E. [DOI] [PubMed] [Google Scholar]
- 45.Bick I.R., Rhee C. Anthraquinone pigments from Phoma foveata Foister. Biochem. J. 1966;98:112–116. doi: 10.1042/bj0980112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quereshi S., Khan N.A., Pandey A.K. Anthraquinone pigment with herbicidal potential from Phoma herbarum FGCC# 54. Chem. Nat. Compd. 2011;47:521–523. [Google Scholar]
- 47.Zheng C.J., Shao C.L., Guo Z.Y., Chen J.F., Deng D.S., Yang K.L., Chen Y.Y., Fu X.M., She Z.G., Lin Y.C., et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012;75:189–197. doi: 10.1021/np200766d. [DOI] [PubMed] [Google Scholar]
- 48.Niu C., Cai M., Zhang Y., Zhou X. Biosynthetic origin of the carbon skeleton of a novel anti-tumor compound, haloroquinone, from a marine-derived fungus, Halorosellinia sp. Biotechnol. Lett. 2012;34:2119–2124. doi: 10.1007/s10529-012-1019-z. [DOI] [PubMed] [Google Scholar]
- 49.Isaka M., Palasarn S., Tobwor P., Boonruangprapa T., Tasanathai K. Bioactive anthraquinone dimers from the leafhopper pathogenic fungus Torrubiella sp. BCC 28517. J. Antibiot. 2012;65:571–574. doi: 10.1038/ja.2012.76. [DOI] [PubMed] [Google Scholar]
- 50.Ayers S., Graf T.N., Adcock A.F., Kroll D.J., Shen Q., Swanson S.M., Matthew S., Carcache de Blanco E.J., Wani M.C., D’Arveaux B.A., et al. Cytotoxic xanthone–anthraquinone heterodimers from an unidentified fungus of the order Hypocreales (MSX 17022) J. Antibiot. 2012;65:3–8. doi: 10.1038/ja.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khamthong N., Rukachaisirikul V., Phongpaichit S., Preedanon S., Sakayaroj J. Bioactive polyketides from the sea fan-derived fungus Penicillium citrinum PSU-F51. Tetrahedron. 2012;68:8245–8250. doi: 10.1016/j.tet.2012.07.060. [DOI] [Google Scholar]
- 52.Zhou X.M., Zheng C.J., Chen G.Y., Song X.P., Han C.R., Li G., Li G.N., Fu Y.H., Chen W.H., Niu Z.G. Bioactive anthraquinone derivatives from the mangrove-derived fungus Stemphylium sp. 33231. J. Nat. Prod. 2014;77:2021–2028. doi: 10.1021/np500340y. [DOI] [PubMed] [Google Scholar]
- 53.Li H., Wei J., Pan S.Y., Gao J.M., Tian J.M. Antifungal, phytotoxic and toxic metabolites produced by Penicillium purpurogenum. Nat. Prod. Res. 2014;28:2358–2361. doi: 10.1080/14786419.2014.940586. [DOI] [PubMed] [Google Scholar]
- 54.Shao C., Wang C., Wei M., Li S., She Z., Gu Y., Lin Y. Structural and spectral assignments of six anthraquinone derivatives from the mangrove fungus (ZSUH-36) Magn. Reson. Chem. 2008;46:886–889. doi: 10.1002/mrc.2266. [DOI] [PubMed] [Google Scholar]
- 55.Shao C., She Z., Guo Z., Peng H., Cai X., Zhou S., Gu Y., Lin Y. 1H and 13C NMR assignments for two anthraquinones and two xanthones from the mangrove fungus (ZSUH-36) Magn. Reson. Chem. 2007;45:434–438. doi: 10.1002/mrc.1974. [DOI] [PubMed] [Google Scholar]
- 56.Miao F.P., Li X.D., Liu X.H., Cichewicz R.H., Ji N.Y. Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs. 2012;10:131–139. doi: 10.3390/md10010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gui R.-Y., Xu L., Kuang Y., Chuang M., III, Qin J.-C., Liu L., Yang S.-X., Zhao L.-C. Chaetominine, (+)-alantrypinone, questin, isorhodoptilometrin, and 4-hydroxybenzaldehyde produced by the endophytic fungus Aspergillus sp. YL-6 inhibit wheat (Triticum aestivum) and radish (Raphanus sativus) germination. J. Plant Interact. 2015;10:87–92. [Google Scholar]
- 58.Kimura Y., Kozawa M., Baba K., Hata K. New constitutents of roots of Polygonum cuspidatum. Planta Med. 1983;48:164–168. doi: 10.1055/s-2007-969914. [DOI] [PubMed] [Google Scholar]
- 59.Ren H., Tian L., Gu Q., Zhu W. Secalonic acid D: A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch. Pharmacal. Res. 2006;29:59–63. doi: 10.1007/BF02977469. [DOI] [PubMed] [Google Scholar]
- 60.Wu B., Wiese J., Wenzel-Storjohann A., Malien S., Schmaljohann R., Imhoff J.F. Engyodontochones, antibiotic polyketides from the marine fungus Engyodontium album strain LF069. Chem. Eur. J. 2016;22:7452–7462. doi: 10.1002/chem.201600430. [DOI] [PubMed] [Google Scholar]
- 61.Huang R., Wang T., Xie X.S., Ma K.X., Fang X.W., Wu S.H. Secondary metabolites from an endophytic fungus Nigrospora sp. Chem. Nat. Compd. 2016;52:697–699. doi: 10.1007/s10600-016-1745-x. [DOI] [Google Scholar]
- 62.Zaidi J.H., Naeem F., Iqbal R., Choudhary M.I., Khan K.M., Perveen S., Shah S.T.A., Hayat S., Voelter Z. Synthesis and bioactivities of naturally occurring anthraquinones: Isochrysophanol, isozyganein, ω-hydroxyisochrysophanol and morindaparvin. Naturforsch. B. 2001;56:689–696. doi: 10.1515/znb-2001-0717. [DOI] [Google Scholar]
- 63.Wang W., Liao Y., Tang C., Huang X., Luo Z., Chen J., Cai P. Cytotoxic and antibacterial compounds from the coral-derived fungus Aspergillus tritici SP2-8-1. Mar. Drugs. 2017;15:348. doi: 10.3390/md15110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee C., Kim S., Li W., Bang S., Lee H., Lee H.J., Noh R.Y., Park J.E., Bang W.Y., Shim S.H. Bioactive secondary metabolites produced by an endophytic fungus Gaeumannomyces sp. JS0464 from a maritime halophyte Phragmites communis. J. Antibiot. 2017;70:737–742. doi: 10.1038/ja.2017.39. [DOI] [PubMed] [Google Scholar]
- 65.Ayer W.A., Trifonov L.S. Anthraquinones and a 10-hydroxyanthrone from Phialophora alba. J. Nat. Prod. 1994;57:317–319. doi: 10.1021/np50104a021. [DOI] [Google Scholar]
- 66.Gill M., Morgan P.M. New fungal anthraquinones. Arkivoc. 2001;7:145–156. [Google Scholar]
- 67.Yang Y., Yan Y.M., Wei W., Luo J., Zhang L.S., Zhou X.J., Wanga P.C., Yang Y.X., Cheng Y.X. Anthraquinone derivatives from Rumex plants and endophytic Aspergillus fumigatus and their effects on diabetic nephropathy. Bioorg. Med. Chem. Lett. 2013;23:3905–3909. doi: 10.1016/j.bmcl.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 68.Na M., Jin W.Y., Min B.S., Ahn J.S., Bae K.H. Protein tyrosine phosphatase 1B inhibitory activity of anthraquinones and stilbenes. Nat. Prod. Sci. 2008;14:143–146. [Google Scholar]
- 69.Zhang Y., Jia A., Chen H., Wang M., Ding G., Sun L., Dai M. Anthraquinones from the saline-alkali plant endophytic fungus Eurotium rubrum. J. Antibiot. 2017;70:1138–1141. doi: 10.1038/ja.2017.121. [DOI] [PubMed] [Google Scholar]
- 70.Anke H., Kolthoum I., Laatsch H. Metabolic products of microorganisms. 192. The anthraquinones of the Aspergillus glaucus group. II. Biological activity. Arch. Microbiol. 1980;126:231–236. doi: 10.1007/BF00409925. [DOI] [PubMed] [Google Scholar]
- 71.Sun P., Huo J., Kurtán T., Mándi A., Antus S., Tang H., Draeger S., Schulz B., Hussain H., Khron K., et al. Structural and stereochemical studies of hydroxyanthraquinone derivatives from the endophytic fungus Coniothyrium sp. Chirality. 2013;25:141–148. doi: 10.1002/chir.22128. [DOI] [PubMed] [Google Scholar]
- 72.Qin S.D., Wang Y., Wang W., Zhu W.M. Anti-H1N1-virus secondary metabolites from mangrove-derived aciduric fungus Penicillium sp. OUCMDZ-4736. Chin. J. Mar. Drugs. 2016;35:21–28. [Google Scholar]
- 73.Jin Y., Qin S., Gao H., Zhu G., Wang W., Zhu W., Wang Y. An anti-HBV anthraquinone from aciduric fungus Penicillium sp. OUCMDZ-4736 under low pH stress. Extremophiles. 2018;22:39–45. doi: 10.1007/s00792-017-0975-6. [DOI] [PubMed] [Google Scholar]
- 74.Anisha C., Sachidanandan P., Radhakrishnan E.K. Endophytic Paraconiothyrium sp. from Zingiber officinale Rosc. displays broad-spectrum antimicrobial activity by production of danthron. Curr. Microbiol. 2018;75:343–352. doi: 10.1007/s00284-017-1387-7. [DOI] [PubMed] [Google Scholar]
- 75.Awad N.E., Kassem H.A., Hamed M.A., El-Naggar M.A.A., El-Feky A.M.M. Bioassays guided isolation of compounds from Chaetomium globosum. J. Mycol. Med. 2014;24:e35–e42. doi: 10.1016/j.mycmed.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 76.Lu H.F., Wang H.L., Chuang Y.Y., Tang Y.J., Yang J.S., Ma Y.S., Chiang J.H., Lu C.C., Yang J.L., Lai T.Y., et al. Danthron induced apoptosis through mitochondria- and caspase-3-dependent pathways in human brain Glioblastoma multiforms GBM 8401 cells. Neurochem. Res. 2009;35:390–398. doi: 10.1007/s11064-009-0067-9. [DOI] [PubMed] [Google Scholar]
- 77.Khan N., Afroz F., Begum M.N., Rony S.R., Sharmin S., Moni F., Choudhury M., Hasand K., Shahaa M., Sohrab M.H. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol. Rep. 2018;5:970–976. doi: 10.1016/j.toxrep.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Masi M., Nocera P., Zonno M.C., Tuzi A., Pescitelli G., Cimmino A., Boari A., Infantino A., Vurro M., Evidente A. Lentiquinones A, B, and C, phytotoxic anthraquinone derivatives isolated from Ascochyta lentis, a pathogen of lentil. J. Nat. Prod. 2018;81:2700–2709. doi: 10.1021/acs.jnatprod.8b00556. [DOI] [PubMed] [Google Scholar]
- 79.Andolfi A., Cimmino A., Villegas-Fernandez A.M., Tuzi A., Santini A., Melck D., Rubiales D., Evidente A. Lentisone, a new phytotoxic anthraquinone produced by Ascochyta lentis, the causal agent of Ascochyta Blight in Lens culinaris. J. Agric. Food Chem. 2013;61:7301–7308. doi: 10.1021/jf4026663. [DOI] [PubMed] [Google Scholar]
- 80.Huang H., Wang F., Luo M., Chen Y., Song Y., Zhang W., Zhang S., Ju J. Halogenated anthraquinones from the marine-derived fungus Aspergillus sp. SCSIO F063. J. Nat. Prod. 2012;75:1346–1352. doi: 10.1021/np3002699. [DOI] [PubMed] [Google Scholar]
- 81.Pan D., Zhang X., Zheng H., Zheng Z., Nong X., Liang X., Ma X., Qi S. Novel anthraquinone derivatives as inhibitors of protein tyrosine phosphatases and indoleamine 2,3-dioxygenase 1 from the deep-sea derived fungus Alternaria tenuissima DFFSCS013. Org. Chem. Front. 2019;6:3252–3258. doi: 10.1039/C9QO00775J. [DOI] [Google Scholar]
- 82.Padhi S., Masi M., Cimmino A., Tuzi A., Jena S., Tayung K., Evidente A. Funiculosone, a substituted dihydroxanthene-1,9-dione with two of its analogues produced by an endolichenic fungus Talaromyces funiculosus and their antimicrobial activity. Phytochemistry. 2019;157:175–183. doi: 10.1016/j.phytochem.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 83.Masi M., Reveglia P., Baaijens-Billones R., Górecki M., Pescitelli G., Savocchia S., Evidente A. Phytotoxic metabolites from three Neofusicoccum species causal agents of Botryosphaeria dieback in australia, luteopyroxin, neoanthraquinone, and luteoxepinone, a disubstituted furo-α-pyrone, a hexasubstituted anthraquinone, and a trisubstituted oxepi-2-one from Neofusicoccum luteum. J. Nat. Prod. 2020;83:453–460. doi: 10.1021/acs.jnatprod.9b01057. [DOI] [PubMed] [Google Scholar]
- 84.Bien H.S., Stawitz J., Wunderlich K. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag; Weinheim, Germany: 2000. Anthraquinone dyes and intermediates. [Google Scholar]
- 85.Vankar P.S., Shanker R., Mahanta D., Tiwari S.C. Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn using biomordant. Dyes Pigments. 2008;76:207–212. doi: 10.1016/j.dyepig.2006.08.023. [DOI] [Google Scholar]
- 86.Akilandeswari P., Pradeep B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016;100:1631–1643. doi: 10.1007/s00253-015-7231-8. [DOI] [PubMed] [Google Scholar]
- 87.Guinot P., Rogé A., Gargadennec A., Garcia M., Dupont D., Lecoeur E., Candeliera L., Andary C. Dyeing plants screening: An approach to combine past heritage and present development. Color Technol. 2006;122:93–101. doi: 10.1111/j.1478-4408.2006.00015.x. [DOI] [Google Scholar]
- 88.Moiseeva Z.Z. Means for preparing and using nitro derivatives of anthraquinone in dye synthesis. Organicheskie Poluprodukty i Krasiteli. 1969;4:70–79. (In Russian) [Google Scholar]
- 89.Salabert J., Sebastián R.M., Vallribera A. Anthraquinone dyes for superhydrophobic cotton. Chem. Commun. 2015;51:14251–14254. doi: 10.1039/C5CC06028A. [DOI] [PubMed] [Google Scholar]
- 90.Benkhaya S., Mrabet S., El Harfi A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020;115:107891. doi: 10.1016/j.inoche.2020.107891. [DOI] [Google Scholar]
- 91.Ledakowicz S., Solecka M., Zylla R. Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J. Biotechnol. 2001;89:175–184. doi: 10.1016/S0168-1656(01)00296-6. [DOI] [PubMed] [Google Scholar]