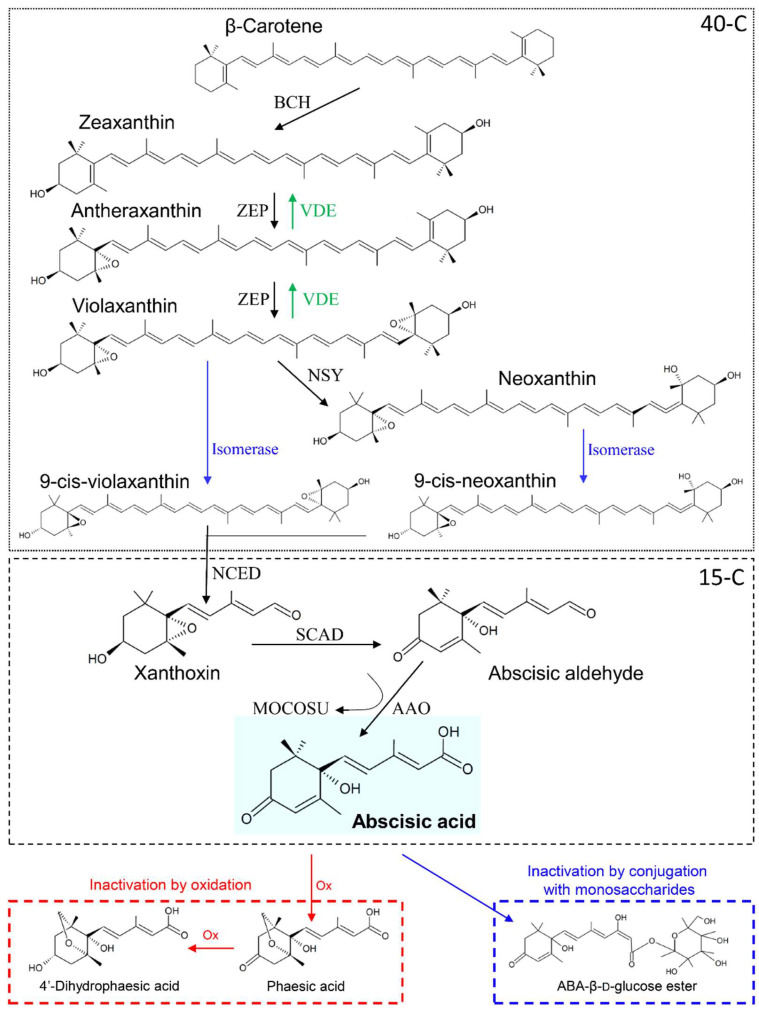
Figure 1.
Abscisic acid biosynthesis and metabolism. In the first step, β-carotene is di-hydroxylated by β-carotene hydroxilase (BCH) proteins to produce the transisomer zeaxanthin. Hence, zeaxanthin is epoxidated by zeaxanthin oxidase (ZEP) to antheraxanthin and, then, to violaxanthin. ZEP-mediated reactions can be reversed by violaxanthin de-epoxidase (VDE). Violaxanthin can be transformed into neoxanthin by neoxanthin synthase (NSY) and both violaxanthin and neoxanthin are converted in the respective 9-cis-isomer by isomerase catalysts. The 15-carbons apocarotenoid sesquiterpenoid xanthoxin is then produced by cis-xanthophylls cleavage, whose reaction is catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED). Subsequently, xanthoxin is oxidized to abscisic aldehyde by short-chain alcohol dehydrogenase (SCAD), and finally abscisic acid (ABA) is produced by oxidation of abscisic aldehyde through the combined action of ABA-aldehyde oxidase (AAO) and a molybdenum cofactor sulfurase (MOCOSU). ABA can be inactivated by oxidation or by conjugation with monosaccharides. In the first way, ABA is oxidized (Ox) at first to phaseic acid and then to 4′-dihydrophaseic acid. In the second one, ABA is conjugated with glucose to produce ABA-β-D-glucose ester. Based on [9].