Figure 3.
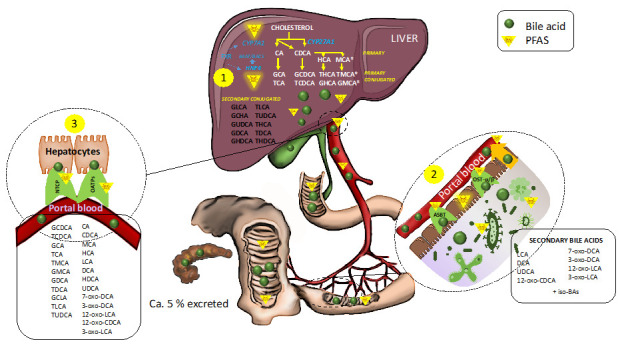
Bile acid biosynthesis and enterohepatic circulation, and the impact of polyfluoroalkyl substances (PFASs) on bile acid (BA) metabolism. (1) In the liver, the classic BA synthesis pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1) which is downregulated by PFAS. The alternative BA synthesis pathway is initiated by CYP27A1 to synthesize primary bile acids, CA and CDCA, in hepatocytes. CDCA can be further converted to HCA and MCA in the liver. CYP7A1 is downregulated by PFAS. Bile acids are conjugated to the amino acids taurine or glycine before being released into the intestine. HNF4α, which plays a central role in bile acid conjugation by direct regulation of VLACSR and BAAT, can be suppressed by PFAS. (2) BAs are recovered into portal blood through a combination of passive absorption in the proximal small intestine, active transport via apical bile salt transporter (ASBT) in the distal ileum, and passive absorption in the colon and via organic solute transporter α/β (OSTα/β). Perfluorooctanesulfonic acid (PFOS) can also be transported by ASBT and OSTα/β. Furthermore, PFAS can increase the permeability of the gut, thus impacting the passive transport pathway of BAs. In the colon, BAs are also deconjugated by bacterial bile salt hydrolase and are 7α-dehydroxylated by bacterial 7α-dehydroxylase to form secondary BAs. PFAS can modify gut microbial composition and thus impact microbial BA formation. (3) BAs are eventually recycled from portal blood back to hepatocytes via Na-taurocholate co-transport peptide (NTCP) and the sodium-independent organic anion transporting polypeptide (OATP). PFASs also utilize the NTCP and OATP transporter. The majority (90–95%) of BAs secreted into the small intestine are actively reabsorbed in the terminal ileum and circulate back to the liver while ca. 5% are excreted via feces.
