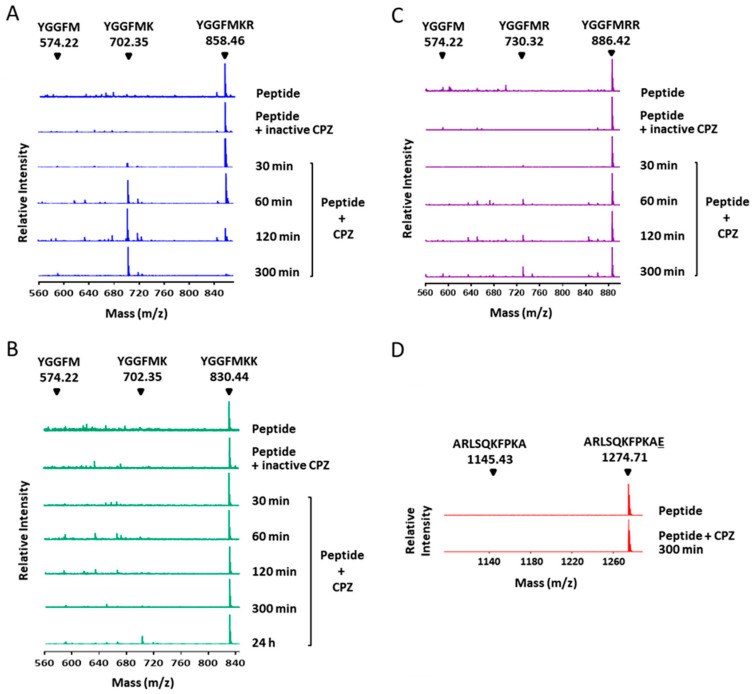
Figure 7.
Substrate specificity of CPZ against Met-enkephalin-derived peptides. MALDI-TOF MS spectra of Met-enkephalin-derived peptides (A) YGGFMKR, (B) YGGFMKK, and (C) YGGFMRR treated with 100 nM CPZ at 37 °C with different incubation times. The peaks with a mass of 858.46 Da, 830.44 Da and 886.42 Da correspond to the peptides YGGFMKR (theoretical monoisotopic MH+ mass = 858.41 Da), YGGFMKK (theoretical monoisotopic MH+ mass = 830.41 Da) and YGGFMRR (theoretical monoisotopic MH+ mass = 886.42 Da) respectively. The peak with a mass of 702.35 Da generated in the presence of CPZ corresponds to the peptide YGGFMK (theoretical monoisotopic MH+ mass = 702.31 Da) produced by the cleavage of the C-terminal Arg or Lys amino acids from the peptides YGGFMKR and YGGFMKK, respectively. The peak with a mass of 730.32 Da generated in the presence of CPZ corresponds to the peptide YGGFMR (theoretical monoisotopic MH+ mass = 730.32 Da) produced by the cleavage of the C-terminal Arg from the peptide YGGFMRR. The position for the peptide YGGFM (with mass of 574.22 Da) is indicated. CPZ was inactivated by incubating the enzyme for 15 min at 90 °C to be used as a control reaction (peptide + inactive CPZ). Control samples were incubated for 24 h at 37 °C; (D) Spectra of the synthetic peptide ARLSQKFPKAE after 300 min of incubation in the absence (peptide) or in the presence of 100 nM CPZ (peptide + CPZ). Numbers above the major peaks indicate the monoisotopic masses of the MH+ ion (m/z) for the full-length peptide (mass = 1274.71 Da) or the peptide without its C-terminal residue, ARLSQKFPKA (mass = 1145.43 Da).