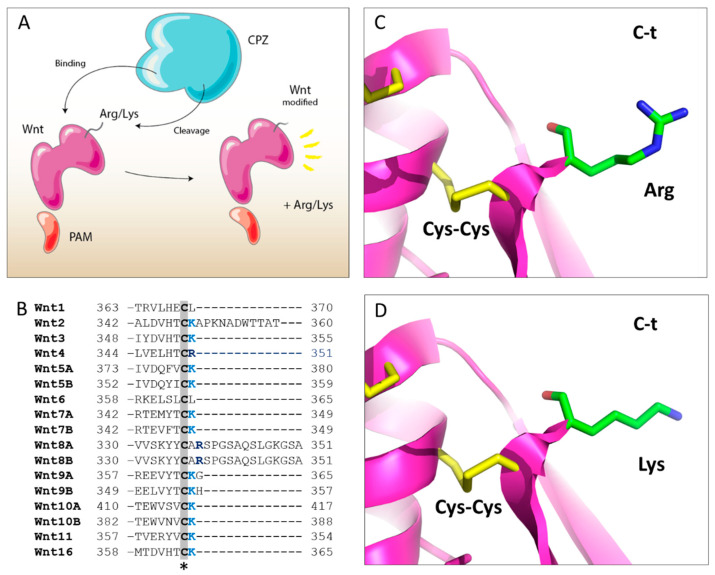
Figure 10.
Comparison of the C-terminal region of human Wnt proteins. (A) Proposed model for the proteolytic cleavage of Wnt proteins by CPZ. (B) Sequence alignment of the C-terminal region of all human Wnt proteins. The star indicates the location of the last conserved cysteine residue, involved in a disulfide bridge formation that links the last two β-sheet regions. The C-terminal Lys and Arg residues located immediately after the last Cys residue in the majority of human Wnt proteins are shown in bright blue or dark blue, respectively; (C,D) Structural modeling of the C-terminal tail of XWnt-8, showing the location of the C-terminal residues identified in the majority of human Wnt proteins. Models were generated by mutating the last Ala residue found in XWnt-8 for Arg or Lys amino acids (C and D, respectively). The last Cys-Cys bond formed in XWnt-8 between Cys295 and Cys337 is indicated.