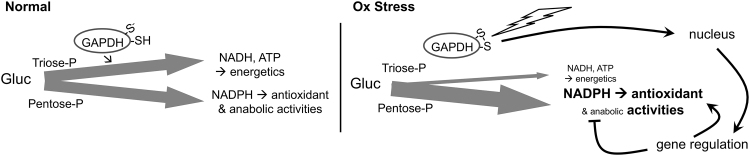
FIG. 5.
Redox sensing and metabolic reprioritization by GAPDH. GAPDH, a critical enzyme on the energy generating triosephosphate pathway, has a highly reactive Cys-thiolate ion in the active site (S−). In normal conditions, GAPDH is active and flux of glucose through the triose-P pathway for energetics versus through the pentose-P pathway for generating NADPH is balanced. During oxidative stress, H2O2 oxidizes the active site thiolate, which inactivates glycolytic activity of GAPDH, lowering glycolytic flux and making more glucose available for the pentose-P pathway; and converts GAPDH into a nuclear-localized transcription factor that further favors antioxidant activities of NADPH arising from the pentose-P pathway. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.