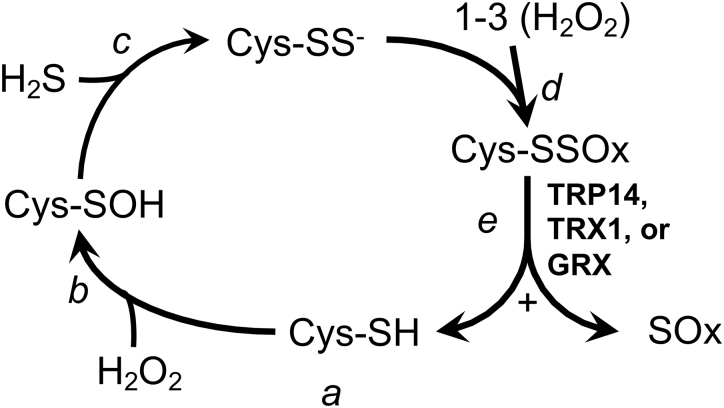
FIG. 6.
Hypothetical persulfidation-based thiol protection. a, In the unstressed state, Cys residues in proteins or metabolites will generally be in the reduced thiol form. b, Exposure to H2O2 yields the unstable sulfenic acid. c, Whereas this will typically react with another thiol to yield a disulfide, in the presence of H2S, a persulfide can form, which will spontaneously ionize to the reactive perthiolate anion at physiological pH. d, The reactive perthiolate anion can react with H2O2, yielding persulfenylate anion. This might further react with H2O2, yielding persulfinylate and persulfonylate anions (in combination, these overoxidized forms designated “-SSOx”). e, Reduction of the disulfide bond in any of the -SSOx species by TRX1, TRP14, or GRX will regenerate the active thiol on the original substrate and liberate an oxidized inorganic S product, designated “SOx.” GRX, glutaredoxin; H2O2, hydrogen peroxide; H2S, hydrogen sulfide; TRP14, thioredoxin-related protein of 14 kDa; TRX1, thioredoxin-1.