Figure 2. Cellular pathogenesis of catecholaminergic polymorphic ventricular tachycardia (CPVT).
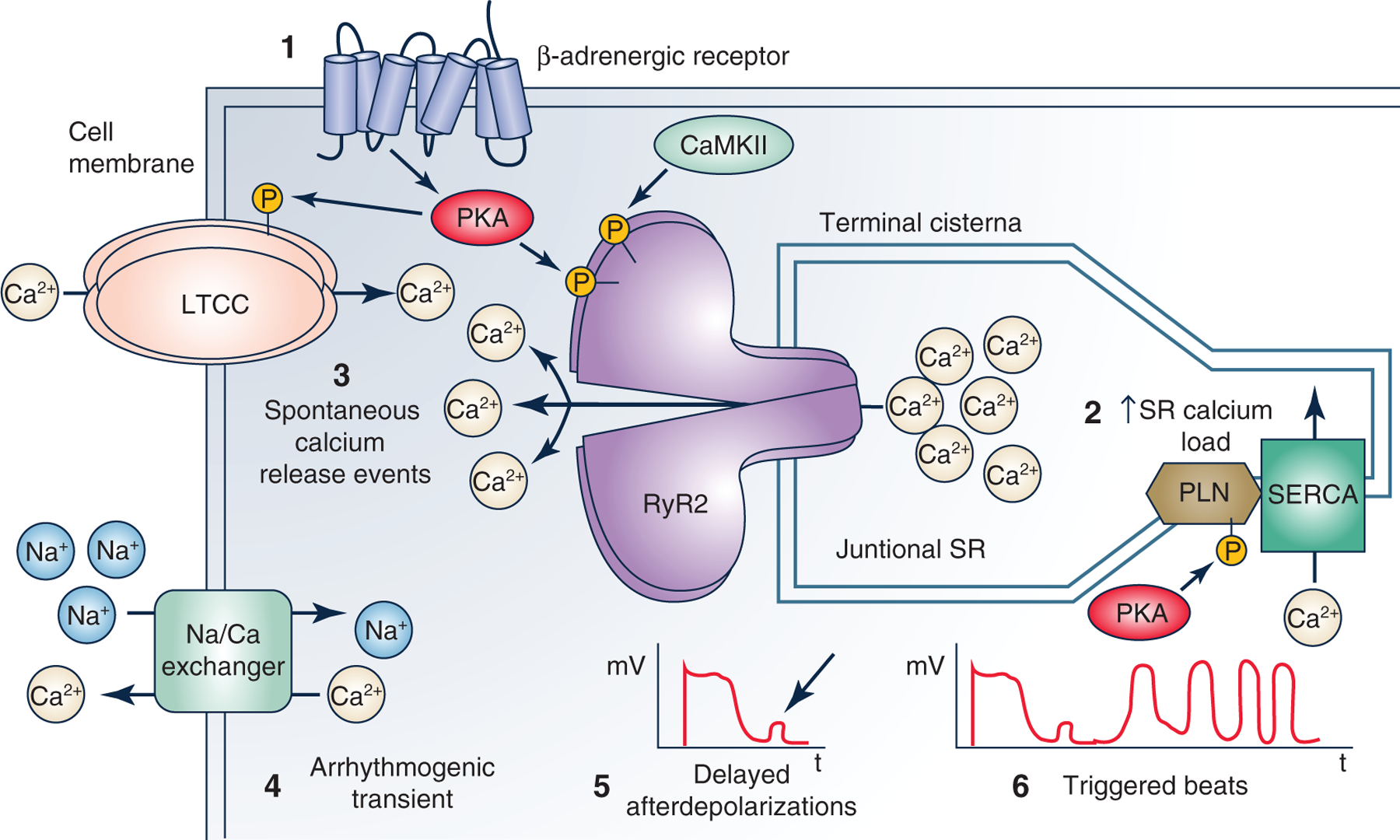
The cartoon illustrates cellular mechanisms underlying CPVT caused by the loss of calsequetrin. 1, catecholamines released during stress or exercise activates β-adrenergic receptor signalling, leading to cardiomyocyte calcium loading and enhanced sarcoplasmic reticulum (SR) Ca uptake. 2, the increased SR calcium load is a physiological response necessary for increasing cardiac output during the physiological fight or flight response (Bers, 2001). Normally, ventricular myocytes can handle the increased SR calcium load. 3, if a CPVT mutation is present, RyR2 SR calcium release channels open spontaneously during late diastole, causing unregulated ‘pathological’ SR calcium release termed ‘spontaneous calcium release events’ (SCR). 4, the rise in cytosolic calcium during the SCR activates the electrogenic sodium calcium exchanger, which generates an arrhythmogenic transient inward current. 5, this induces a cell membrane depolarization termed ‘delayed afterdepolarizations’ (DADs). 6, DADs are a well-established cellular mechanism that can then cause triggered beats that lead to ventricular arrhythmias (Priori & Corr, 1990).
