Figure 3. Summary of current hypotheses for how RyR2 mutations could lead to catecholaminergic polymorphic ventricular tachycardia.
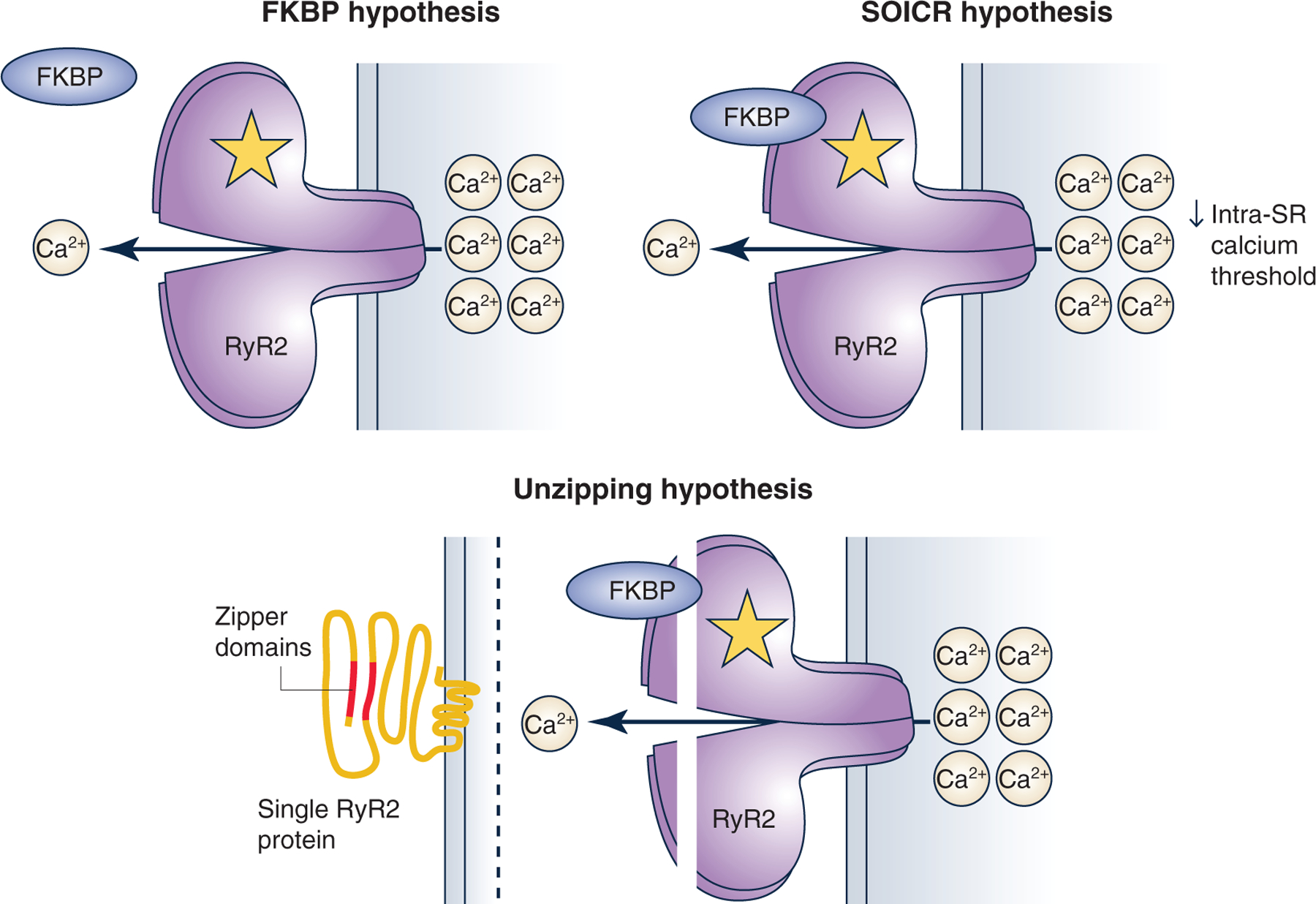
The first theory states that mutations prevent FKBP binding to RyR2. The second theory states that a mutation can lower the intra-sarcoplasmic reticulum (SR) calcium threshold needed RyR2 to open during diastole, termed ‘store overload-induced calcium release’ (SOICR). Finally, the ‘unzipping’ theory stems from the observation that the N-terminal and central domain of RyR2 interact with one another forming a tight seal. Mutations in RYR2 can affect the interaction and lead to an unzipping of the protein, making RyR2 more prone to open spontaneously.
