Figure 1.
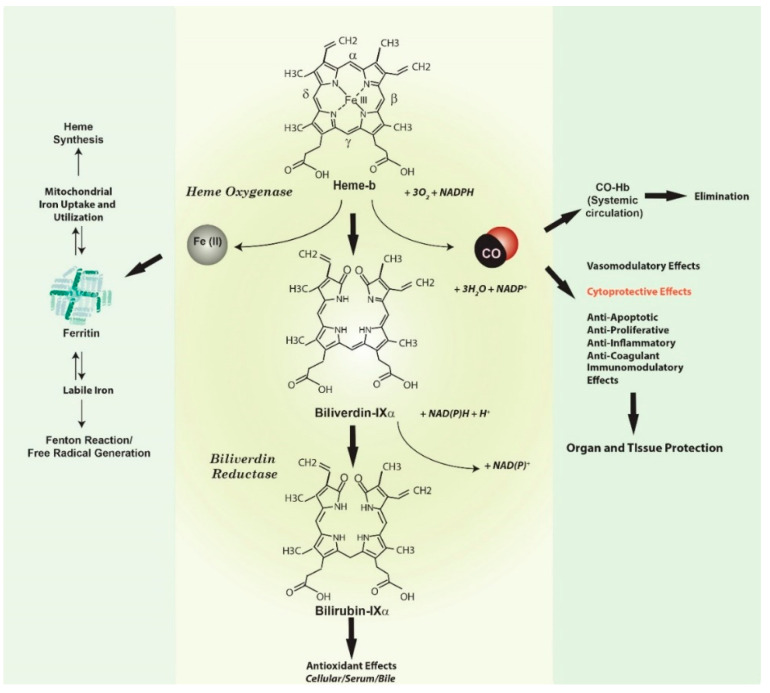
Heme oxygenase activity and end-product generation. Heme oxygenase (HO) is a metabolic enzyme that catalyzes the rate-limiting step in the oxidative catabolism of heme to generate the bile pigment biliverdin-IXα (BV) [1,2]. The HO reaction requires molecular oxygen (O2) and reducing equivalents from NADPH cytochrome p-450 reductase. The HO reaction releases the α-methene bridge carbon of heme as carbon monoxide (CO). The heme iron is released as ferrous iron (Fe II). BV is further metabolized to bilirubin-IXα (BR) by NAD(P)H biliverdin reductase [1,2,3]. Both BV and BR are potent antioxidants [22,23,24]. The metabolic fate of iron released by HO activity remains incompletely understood. Iron may act as a catalyst for the Haber-Weiss cycle, leading to the production of the highly reactive and deleterious hydroxyl radical, and may promote other free radical generating reactions such as the decomposition of organic peroxides [27]. Seminal studies suggested that HO-derived iron can trigger the synthesis of ferritin via modulation of iron binding protein activity [21]. Ferritin can in turn act as a potential cytoprotectant in the endothelium. Endogenously produced HO-1 derived CO has a myriad of possible biological effects, including vasoregulation, as well as cytoprotective effects that include regulation of inflammation, apoptosis, and cell proliferation, and modulation of immune responses [17,18,25,26].