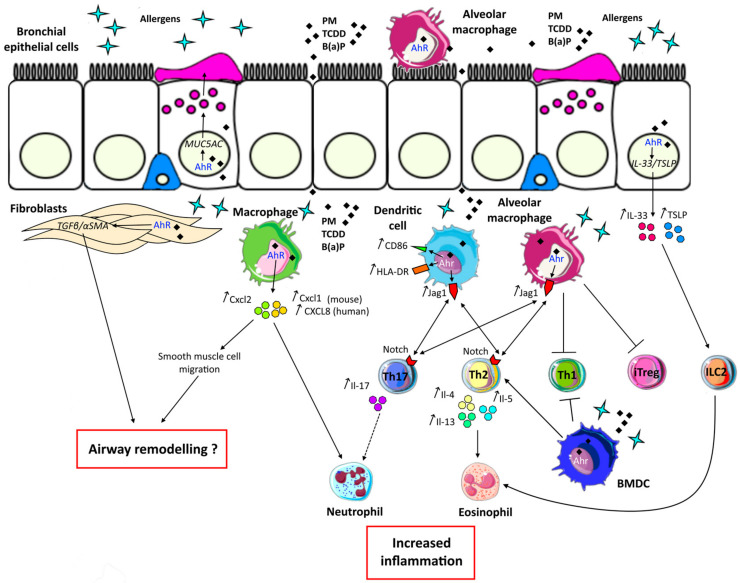
Figure 4.
Deleterious effects of aryl hydrocarbon receptor (AhR) activation in asthma. Bronchial epithelial cells are the first barrier against environmental agents. In asthma, exposure to allergens and environmental AhR ligands such as particulate matter (PM), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) or Benzo(a)pyrene (B(a)P) induces MUC5AC expression leading to mucus hypersecretion through AhR. Moreover, the alarmins interleukin (IL)-33 and Thymic Stromal Lymphopoietin (TSLP) are also induced through this pathway. These cytokines are able to activate innate lymphoid cells type 2 (ILC2) inducing eosinophil recruitment. Other structural cells implicated in asthma and expressing AhR are fibroblasts. Co-activation of these cells by an allergen and AhR ligands leads to TGF-β and α-SMA transcription, which are implicated in airway remodelling. Bone marrow derived-macrophages (BMDM) can also participate in airway remodelling through secretion of Cxcl2, a chemokine induced by Ahr activation and leading to smooth muscle cell migration. Moreover, increased secretion of Cxcl2 as well as Cxcl1 (or CXCL-8/IL-8 in humans) in response to allergen and AhR ligands costimulation leads to the recruitment of neutrophils. Neutrophil inflammation is associated with severe asthma and increased secretion of IL-17. The Th17 pathway is also induced by allergens and AhR ligands activated -dendritic cells and -alveolar macrophages through increased Jag1 expression. Furthermore, alveolar macrophages are implicated in Th1 and induced T regulatory cell (iTreg) inhibition through Ahr activation. In response to Ahr signalling, dendritic cells through increased expression of costimulatory molecule CD86 and HLA-DR, as well as alveolar macrophages participate in increased Thelper (Th)2 activation. Moreover, bone marrow derived-dendritic cells (BMDC) stimulated with an allergen and AhR ligands can amplify type 2 responses by inhibiting Th1 and activating Th2 cells. All these AhR-dependent signals result in increased Th17/neutrophilic and/or Th2/ILC2/eosinophilic inflammation, together with decreased Th1/Treg regulation.