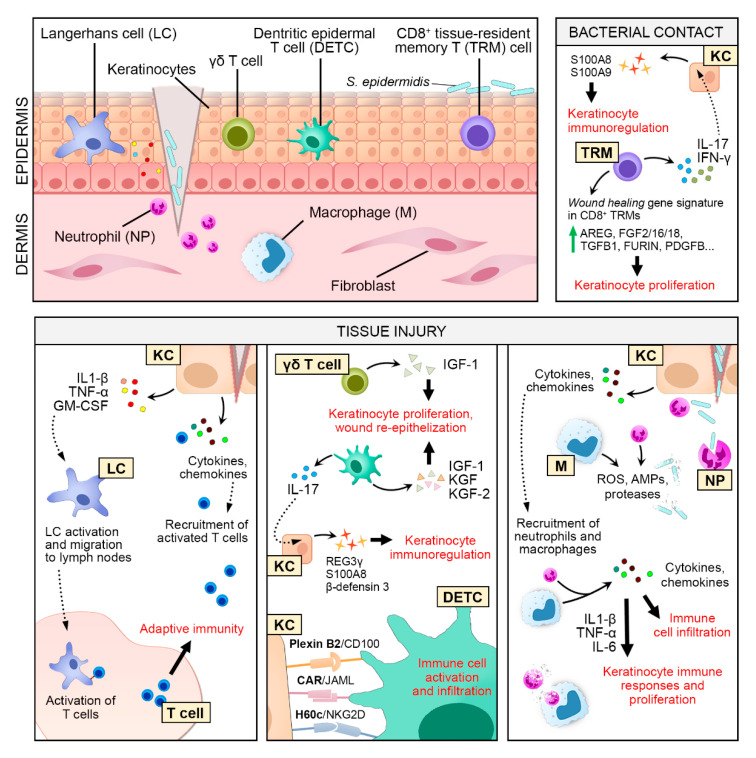
Figure 2.
Keratinocyte‒immune cell crosstalk in skin wound healing. Keratinocyte (KC)-secreted pro-inflammatory cytokines and chemokines contribute to the recruitment of neutrophils (NP) and macrophages (M) to the site of injury. NP and M remove cellular debris and destroy invading pathogens by phagocytosis and the release of reactive oxygen species (ROS), proteases, and antimicrobial proteins (AMPs). They further amplify the inflammatory response by secreting various pro-inflammatory cytokines and chemokines to recruit more immune cells. Some of these secreted factors, such as interleukin 1 beta (IL-1β), tumor necrosis factor (TNF-α), and interleukin 6 (IL-6) stimulate keratinocyte proliferation and immune responses. After tissue injury, Langerhans cells (LC) react to KC-derived factors and migrate to the draining lymph node to activate T cells, triggering an adaptive immune response. KC-derived cytokines and chemokines serve as a chemoattractant to recruit activated T cells to the wound. Epidermal γδ T cells (human) and dendritic epidermal T cells (DETCs, mouse) respond rapidly to tissue injury and produce growth factors to activate KC proliferation and wound re-epithelization. Vice versa, ligands expressed on the KC cell membrane, i.e., Plexin B2, coxsackievirus and adenovirus receptor (CAR), and H60c are detected by DETCs, triggering immune cell activation and infiltration. DETC-produced interleukin 17 (IL-17) also activates KC to produce antimicrobial peptides (AMPs). Even in the absence of tissue injury, bacterial contact with skin commensal Staphylococcus epidermidis induces CD8+ tissue-resident memory T (TRM) cells to produce IL-17 and Interferon gamma (IFN-γ), which in turn activate KC to produce AMPs. S. epidermidis-activated TRM cells upregulate the expression of KC mitogens, such as amphiregulin (AREG), fibroblast growth factor 2 (FGF2), and transforming growth factor beta 1 (TGFB1), to promote keratinocyte proliferation. In the later phase of wound healing, the engulfment of apoptotic NP by M is essential for M’s conversion into an anti-inflammatory phenotype and the production of several key factors for wound healing, including TGFB1, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF).