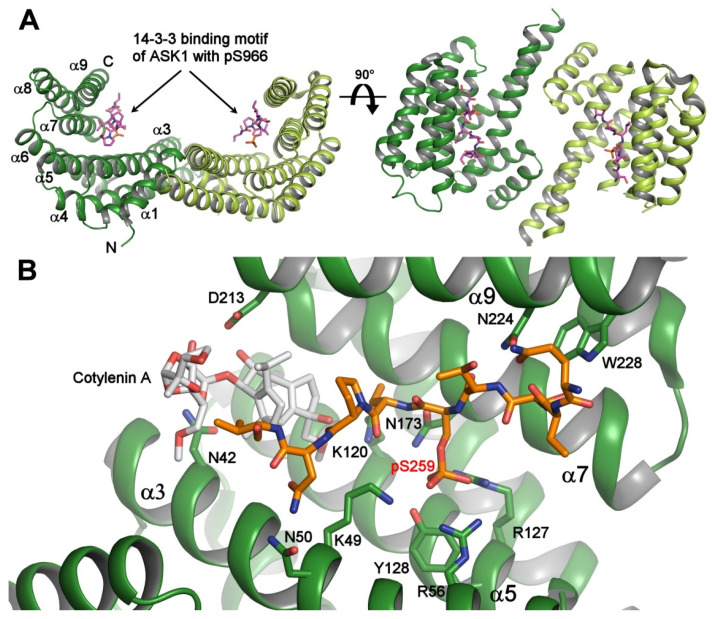
Figure 1.
Crystal structure of the 14-3-3 protein with bound phosphopeptides. (A) The 14-3-3 binding motif of apoptosis signal-regulating kinase 1 (ASK1) (sequence RSIpS966LPVP) bound to human 14-3-3ζ (PDB ID: 6EJL). The 14-3-3 protein molecule is a dimer with a two-fold symmetry, and each protomer consists of nine antiparallel α-helices and contains an amphipathic groove which is a binding site for the phosphorylated motifs; (B) the ternary complex between human 14-3-3σ (shown in green), the 14-3-3 binding motif pSer259 of C-RAF (shown in orange), and Cotylenin A (shown in gray) (PDB ID: 4IHL [21]). The 14-3-3σ residues that make polar contacts with the phosphopeptide and Cotylenin are shown as sticks. The Cotylenin A considerably enhances the binding of the C-RAF pSer259-motif to 14-3-3. The figure was prepared with PyMOL (https://pymol.org/2/).