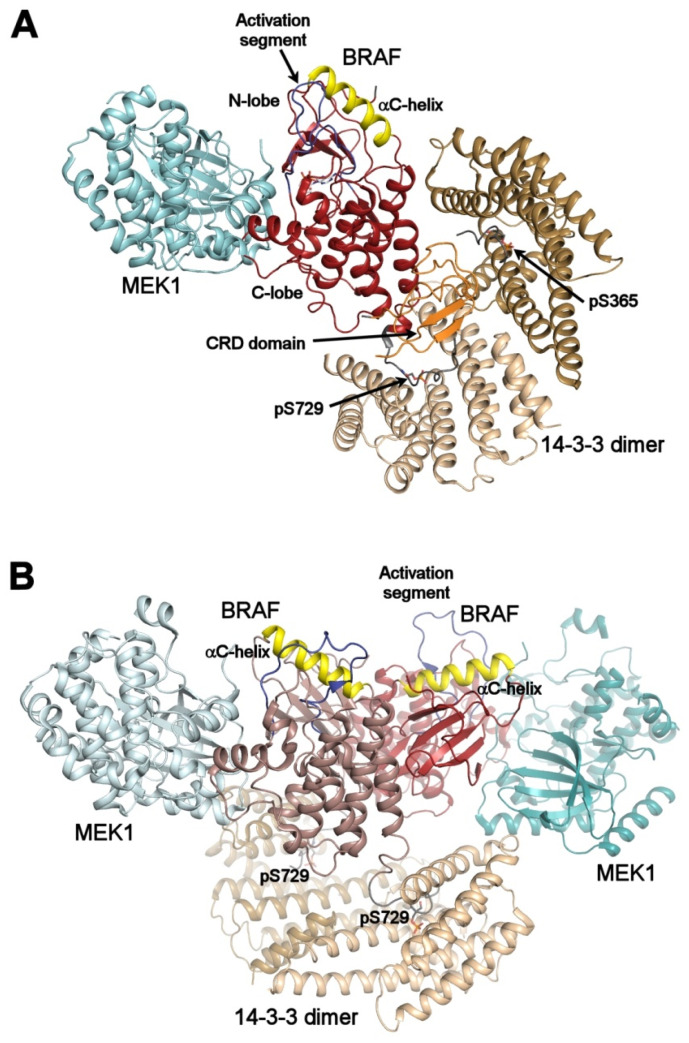
Figure 3.
Autoinhibited and active B-RAF:MEK1:14-3-3 complexes. (A) structure of the autoinhibited BRAF:MEK1:14-3-3 complex (PDB ID: 6NYB [6,7,24,26]). The 14-3-3 dimer simultaneously binds both pSer365 and pSer729 motifs (shown in gray) bordering the B-RAF kinase domain (shown in dark red). The CRD domain (shown in orange) is sequestered within the central channel of the 14-3-3 dimer. The position of the αC-helix (shown in yellow) and the activation segment (shown in blue) correspond to the autoinhibited state. The 14-3-3 protein inhibits B-RAF by blocking its membrane localization dimerization through steric occlusion; (B) structure of the active B-RAF:MEK1:14-3-3 complex (PDB ID: 6Q0J [6,7,24,26]). Dephosphorylation of the N-terminal motif (pSer365) causes structural rearrangement resulting in an active B-RAF dimer stabilized by the 14-3-3 dimer through anchoring C-terminal pSer729 motifs of two B-RAF molecules. The B-RAF kinase domains are oriented in the back-to-back fashion with the αC-helix in a position consistent with the active conformation. The figure was prepared with PyMOL (https://pymol.org/2/).