Table 1.
Structures, Mulliken population values, and HOMO-LUMO energy gap of the synthetic derivatives.
| Derivative 1 | Structure | Mulliken Population Values (Electron Density) | HOMO-LUMO 2 Energy Gap (kcal/mol) | |
|---|---|---|---|---|
| Left | Right | |||
| 6,14′ |
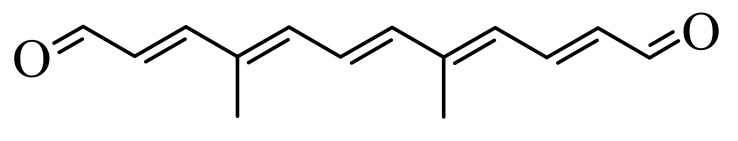
|
6.16 | 6.10 | 189.51 |
| 10,10′ |
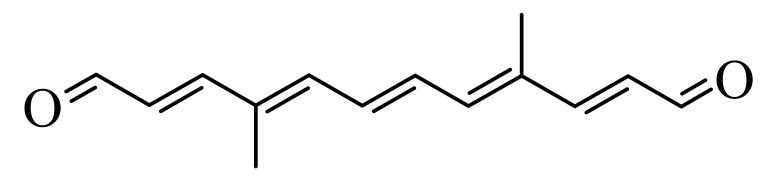
|
6.17 | 6.17 | 191.39 |
| 8,8′ |
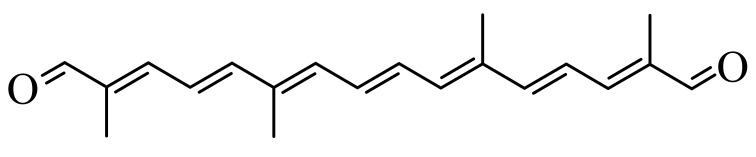
|
6.21 | 6.21 | 178.21 |
| 8,12′ |
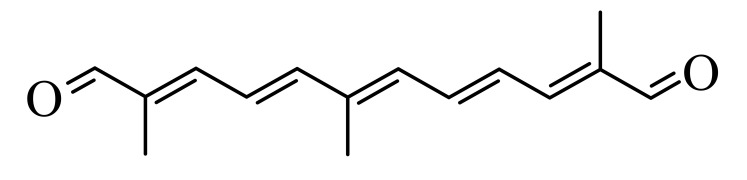
|
6.23 | 6.22 | 210.84 |
| 12,12′ |
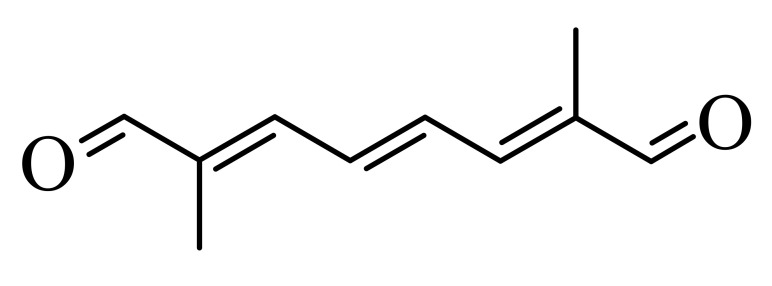
|
6.24 | 6.24 | 214.61 |
1 The abbreviated names of the derivatives are derived from the putative position of oxidative cleavage in the carotenoid backbone, which could lead to the formation of these derivatives, Full names: 6,14′-diapocarotene-6,14′-dial (6,14′); 10,10′-diapocarotene-10,10′-dial (10,10′); 8,8′-diapocarotene-8,8′-dial (8,8′); 8,12′-diapocarotene-8,12′-dial (8,12′); 12,12′-diapocarotene-12,12′-dial (12,12′). 2 HOMO: High Occupied Molecular Orbitals; LUMO: Low Unoccupied Molecular Orbitals.
