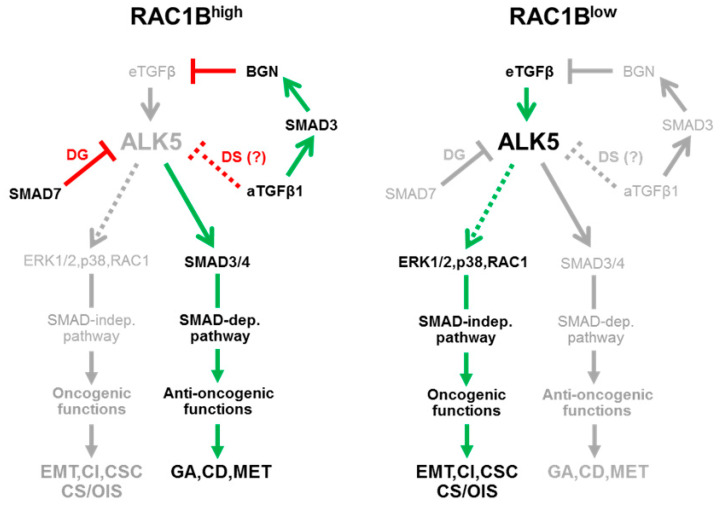
Figure 4.
Schematic diagram illustrating the proposed role of RAC1B in regulating TGFβ signaling in pancreatic tumor cells. Left-hand side, RAC1B inhibits receptor activation via (i) induction of SMAD7 and SMAD7-mediated degradation (DG) of ALK5, (ii) autocrine TGFβ (aTGFβ)-mediated desensitization (DS) of cells towards exogenous TGFβ, and (iii) aTGFβ-SMAD3-mediated induction of BGN, which sequesters exogenous TGFβ (eTGFβ) in the pericellular space and prevents it from binding to the receptors. As a result, the activities of the Smad3/4 and non-Smad (ERK1/2, p38, RAC1) signaling pathways are repressed, but the decrease in Smad signaling is partially rescued by RAC1B-driven upregulation of SMAD3 and SMAD4 (not shown). This constellation suppresses oncogenic programs like EMT, cell invasion (CI), and cancer stem cell (CSC) formation, but allowing tumor-suppressive functions such as growth arrest (GA), cell death/apoptosis (CD), and mesenchymal–epithelial transition (MET) to proceed. Right-hand side, under conditions of low or absent RAC1B expression, ALK5 expression is increased and subsequent activation of non-canonical signaling predominates over Smad signaling, an effect that is enhanced by the inability of the cell to produce additional SMAD3 and SMAD4 proteins. Non-canonical TGFβ signaling pathways, in particular ERK and RAC1, can now promote their oncogenic functions. Cellular senescence (CS) and oncogene-induced senescence (OIS), although driven by MEK-ERK signaling [92,93,94], are generally considered tumor-suppressive mechanisms but may also be tumor-promoting in some instances [112]. Stimulatory interactions are indicated by arrows and inhibitory interactions by lines (green/red = activated, gray-shaded = inactivated). Stippled lines denote still hypothetical interactions (aTGFβ) or the possibility of ALK5-independent activation (ERK1/2, RAC1).